Endocrinol Metab.
2012 Dec;27(4):255-267. 10.3803/EnM.2012.27.4.255.
Vitamin D: A D-Lightful Vitamin for Health
- Affiliations
-
- 1Vitamin D, Skin and Bone Research Laboratory, Section of Endocrinology, Department of Medicine, Nutrition and Diabetes, Boston University Medical Center, Boston, MA, USA. mfholick@bu.edu
- KMID: 1973786
- DOI: http://doi.org/10.3803/EnM.2012.27.4.255
Abstract
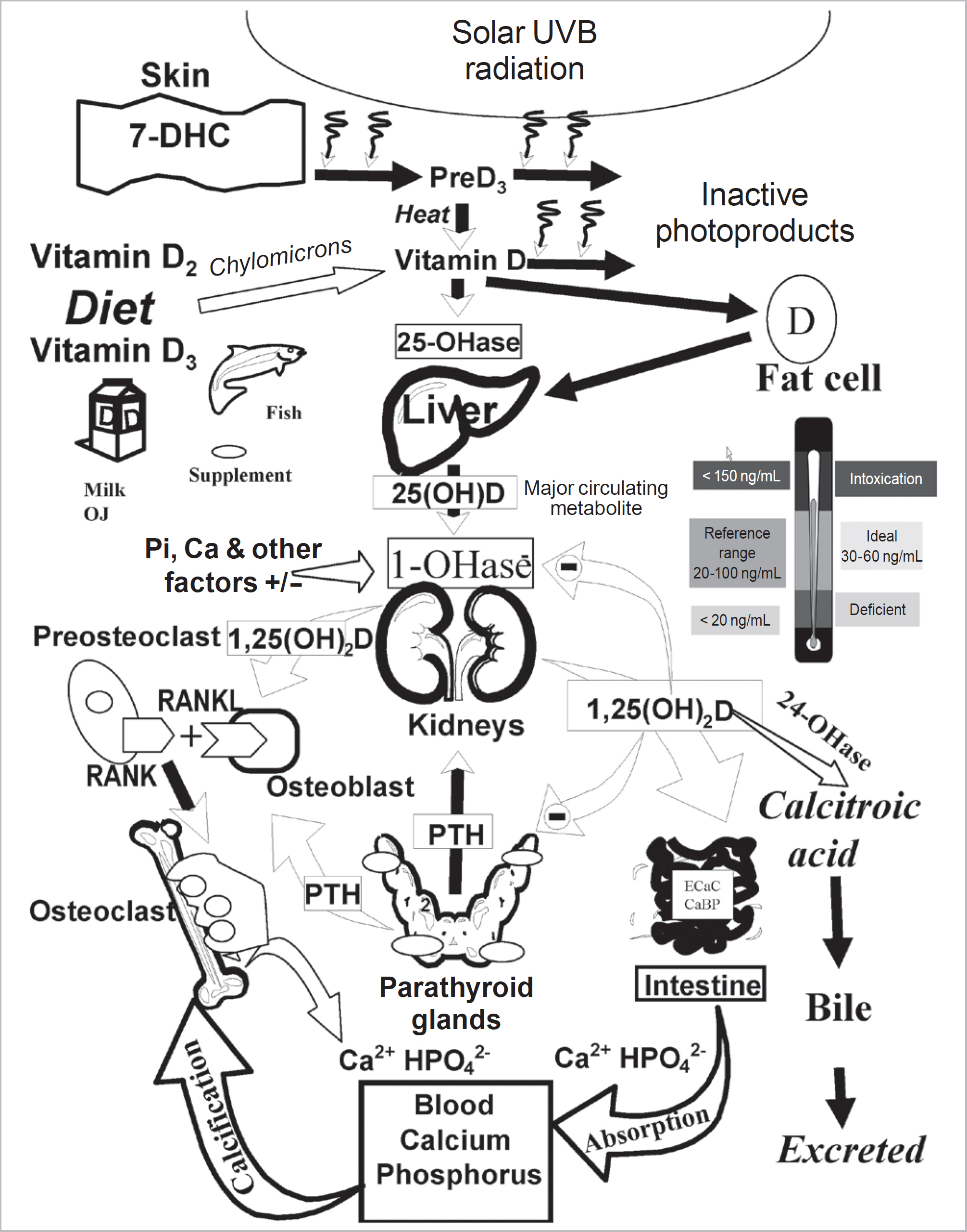
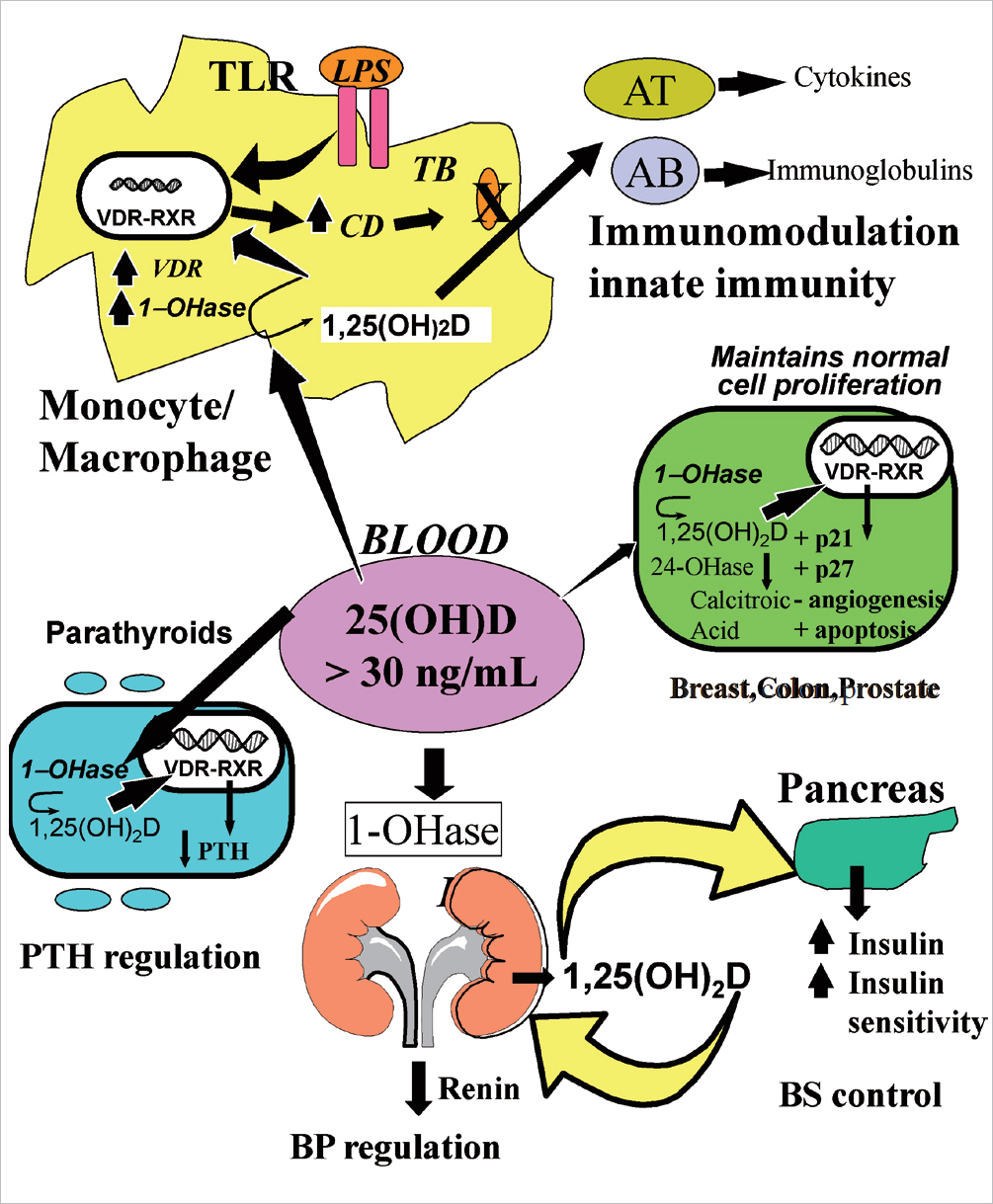
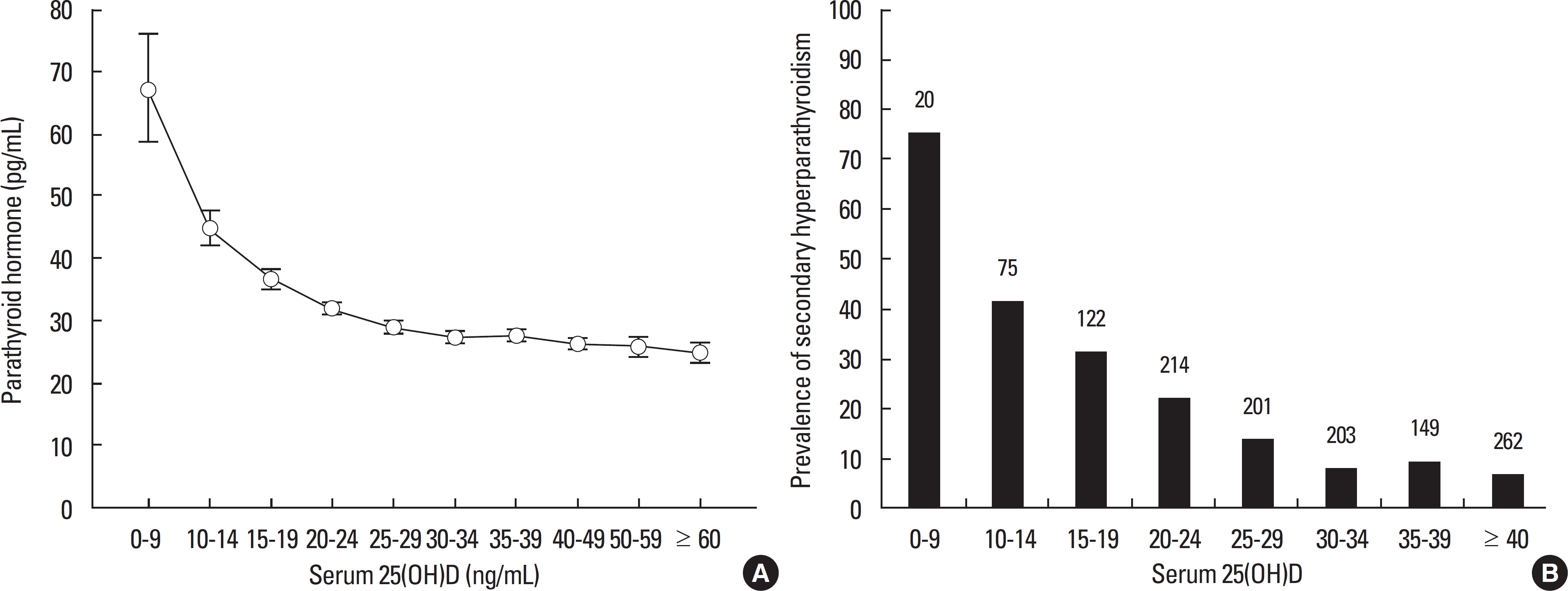
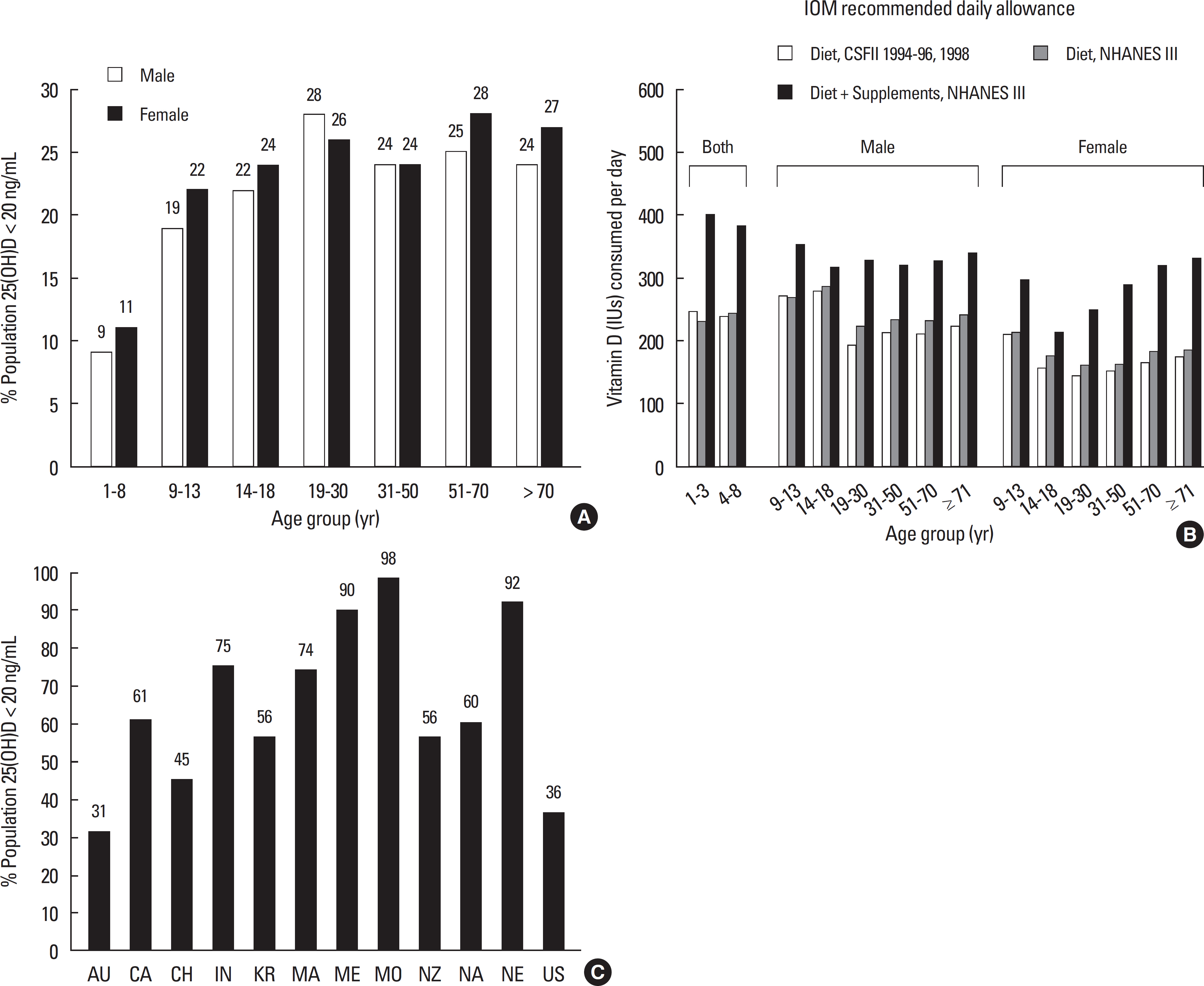
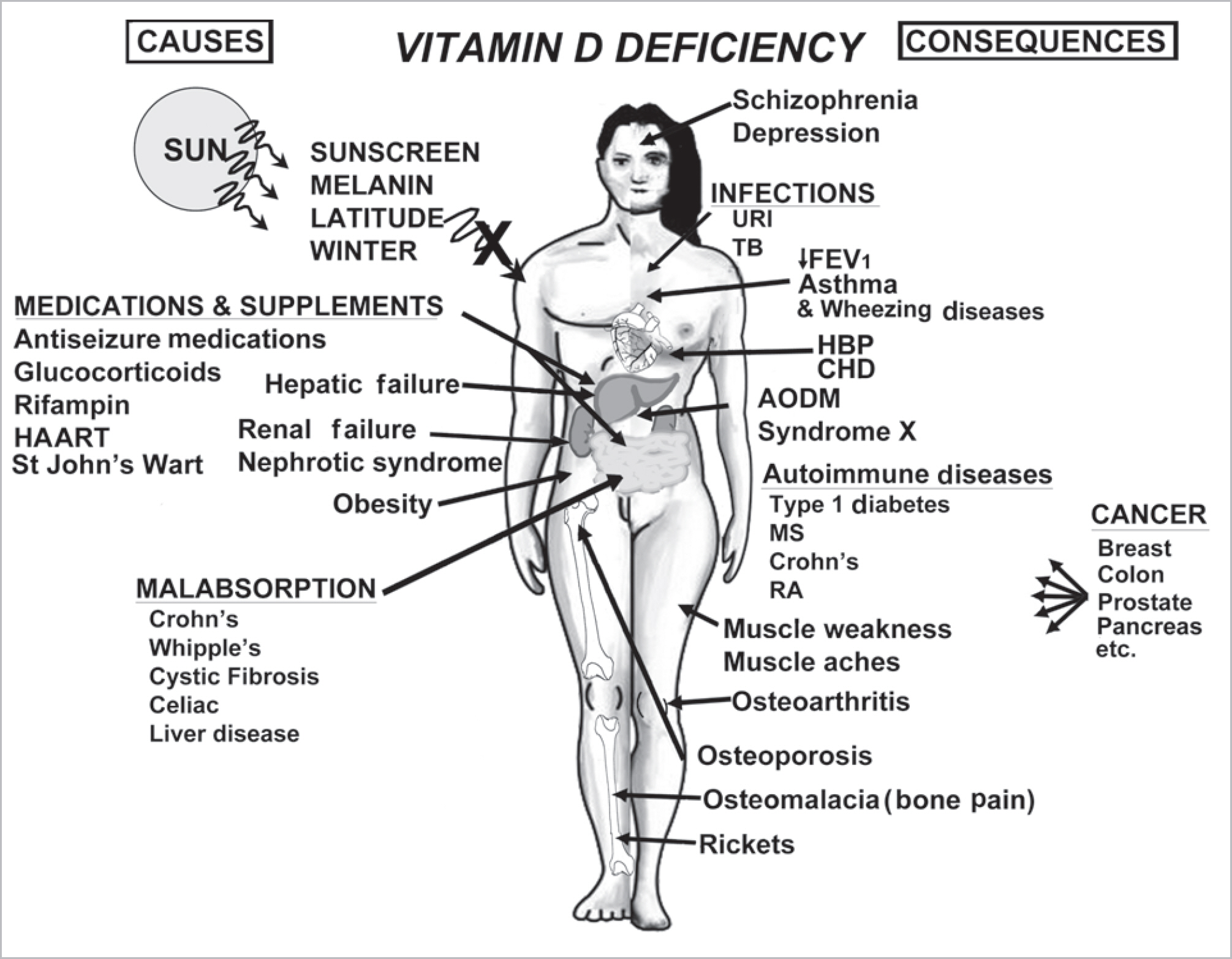
- Vitamin D is a sunshine vitamin that has been produced on this earth for more than 500 million years. Because foods contain so little vitamin D most humans have always depended on sun exposure for their vitamin D requirement. Vitamin D deficiency has been defined as a serum 25-hydroxyvitamin D concentration < 20 ng/mL (50 nmol/L); vitamin D insufficiency as a serum 25-hydroxyvitamin D of 21-29 ng/mL and vitamin D sufficiency as a serum 25-hydroxyvitamin D of 30-100 ng/mL whereas toxicity is usually not seen until blood levels are above 150 ng/mL. Vitamin D deficiency is a global health problem that increases risk for metabolic bone diseases in children and adults as well as many chronic illnesses including autoimmune diseases, type 2 diabetes, cardiovascular disease, infectious disease, and cancer. The major causes of vitamin D deficiency are lack of adequate sensible exposure to sunlight, inadequate dietary intake and obesity. The United States Endocrine Society recommended that to prevent vitamin D deficiency in those at risk, children 1 year and older require 600-1,000 international unit (IU) of vitamin D daily and adults require 1,500-2,000 IU of vitamin D daily. Obese patients require 2-3 times more vitamin D to both treat and prevent vitamin D deficiency.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Korean Society for Bone and Mineral Research Task Force Report: Perspectives on Intermittent High-dose Vitamin D Supplementation
Han Seok Choi, Yong-Ki Min, Dong Won Byun, Myung Hoon Hahn, Kyoung Min Kim, Beom Jun Kim, Ki-Won Oh
J Bone Metab. 2017;24(3):141-145. doi: 10.11005/jbm.2017.24.3.141.Endocrine Risk Factors for Cognitive Impairment
Jae Hoon Moon
Endocrinol Metab. 2016;31(2):185-192. doi: 10.3803/EnM.2016.31.2.185.
Reference
-
1. Holick MF. Phylogenetic and evolutionary aspects of vitamin D from phy-toplankton to humans. Pang PK, Schreibman MP, editors. Vertebrate Endocrinology: Fundamentals and Biomedical Implications. p. 7–43. San Diego: Academic;1989.2. Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 88:296–307. 2003.
Article3. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 116:2062–2072. 2006.
Article4. Huldschinsky K. Heilung von Rachitis durch Kunstliche Hohensonne. Deutsche Med Wochenschr. 45:712–713. 1919.5. Hess AF, Unger LJ. The cure of infantile rickets by sunlight. JAMA. 77:39–41. 1921.
Article6. Steenbock H. The induction of growth promoting and calcifying proper-ties in a ration by exposure to light. Science. 60:224–225. 1924.
Article7. Samuel HS. Infantile hypercalcaemia, nutritional rickets, and infantile scurvy in great Britain. A British Paediatric Association report. Br Med J. 1:1659–1661. 1964.8. Taylor AB, Stern PH, Bell NH. Abnormal regulation of circulating 25-hydroxyvitamin D in the Williams syndrome. N Engl J Med. 306:972–975. 1982.
Article9. Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Ander-son RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 210:203–205. 1980.10. Tian XQ, Chen TC, Matsuoka LY, Wortsman J, Holick MF. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J Biol Chem. 268:14888–14892. 1993.
Article11. Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 91:2552–2555. 1993.
Article12. Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 64:1165–1168. 1987.13. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pig-ment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1:74–76. 1982.
Article14. Holick MF. Vitamin D deficiency. N Engl J Med. 357:266–281. 2007.
Article15. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 96:E447–E452. 2011.
Article16. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Re-itz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 93:677–681. 2008.
Article17. Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 93:2716–2721. 2008.
Article18. Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 88:695–705. 2003.
Article19. Holick MF. The D-lightful vitamin D for child health. JPEN J Parenter Enteral Nutr. 36(1 Suppl):9S–19S. 2012.
Article20. Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 95:471–478. 2010.
Article21. Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 94:26–34. 2009.
Article22. Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for vitamin D. Clin Rev Bone Miner Metab. 7:2–19. 2009.
Article23. Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 26:662–687. 2005.
Article24. Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Na-gai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 19:2685–2695. 2005.
Article25. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Stein-meyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 311:1770–1773. 2006.
Article26. Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1:307–309. 1985.
Article27. Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 103:708–711. 2007.
Article28. Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 94:1867–1875. 2002.
Article29. Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 88:327–331. 2003.
Article30. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 79:820–825. 2004.31. Holick MF. Vitamin D, sunlight and cancer connection. Anticancer Agents Med Chem. In press. 2012.32. Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk fac-tor? J Am Coll Cardiol. 52:1949–1956. 2008.33. Ross AC. Institute of Medicine (U.S.), Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington DC: National Academies Press;2011.34. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 96:1911–1930. 2011.
Article35. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 25:305–312. 2010.36. Maxmen A. Nutrition advice: the vitamin D-lemma. Nature. 475:23–25. 2011.
Article37. Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ. Healthy elderly French women living at home have secondary hyperpara-thyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab. 81:1129–1133. 1996.
Article38. Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 338:777–783. 1998.
Article39. Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 90:3215–3224. 2005.
Article40. Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM. Interventions to raise vitamin D level and functional outcomes: a systematic review and metaanalysis. Poster session presented at: The Americal College of Preventive Medicine Annual Meeting, 2010 Feb 17-20, Arlington, VA.41. Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila). 46:42–44. 2007.
Article42. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 22(Suppl 2):V39–V44. 2007.
Article43. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK. Vitamin D insufficiency in Korea: a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 96:643–651. 2011.44. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 92:3517–3522. 2007.
Article45. Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 94:940–945. 2009.
Article47. Holick MF, Lim R, Dighe AS. Case records of the Massachusetts General Hospital. Case 3-2009. A 9-month-old boy with seizures. N Engl J Med. 360:398–407. 2009.48. Keller KA, Barnes PD. Rickets vs. abuse: a national and international epi-demic. Pediatr Radiol. 38:1210–1216. 2008.
Article49. Bishop NJ, Plotkin H. When is a fracture child abuse? Bone. 23(5 Suppl 1):S458. 1998.50. Kim BG, Chang SK, Kim SM, Hwang JS, Jung JW. Dilated cardiomyop-athy in a 2 month-old infant: a severe form of hypocalcemia with vitamin d deficient rickets. Korean Circ J. 40:201–203. 2010.
Article51. Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 358:1500–1503. 2001.
Article52. Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 51:1391–1398. 2008.
Article53. Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 181-182:71–78. 2002.
Article54. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hy-droxyvitamin D levels and risk of multiple sclerosis. JAMA. 296:2832–2838. 2006.
Article55. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Iowa Women's Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 50:72–77. 2004.
Article56. Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 96:252–261. 2006.
Article57. Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 354:2287–2288. 2006.
Article58. Yamaji T, Iwasaki M, Sasazuki S, Sakamoto H, Yoshida T, Tsugane S. Association between plasma 25-hydroxyvitamin D and colorectal adenoma according to dietary calcium intake and vitamin D receptor polymorphism. Am J Epidemiol. 175:236–244. 2012.
Article59. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 85:1586–1591. 2007.
Article60. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Ben-jamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 117:503–511. 2008.
Article61. Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 28:1179–1185. 2008.62. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 168:1629–1637. 2008.
Article63. Thomas GN, ó Hartaigh B, Bosch JA, Pilz S, Loerbroks A, Kleber ME, Fischer JE, Grammer TB, Böhm BO, März W. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: the Ludwigshafen Risk and Cardiovascular Health (LU-RIC) Study. Diabetes Care. 35:1158–1164. 2012.64. Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab. 97:1953–1961. 2012.
Article65. Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose toler-ance. Diabetologia. 55:1668–1678. 2012.
Article66. Hess AH. Collected writings. 1:p. 669–719. Springfield: Charles C. Thomas;1936.67. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Ran-domized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 91:1255–1260. 2010.
Article68. Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 5:e11088. 2010.
Article69. Reis JP, D. vM, Miller ER 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 124:e371–379. 2009.
Article70. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 124:e362–e370. 2009.
Article71. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 77:204–210. 2003.
Article72. Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 169:1806–1808. 2009.
Article73. Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. Leiden Skin Cancer Study. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 120:1087–1093. 2003.
Article74. New Zealand Ministry of Health, Cancer Society of New Zealand, Acci-dent Compensation Corporation (N.Z.). Consensus statement on vitamin D and sun exposure in New Zealand. Wellington: Ministry of Health;2012.75. Daly RM, Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Sikaris KA, Zimmet PZ, Ebeling PR, Shaw JE. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf). 77:26–35. 2012.76. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 97:1153–1158. 2012.
Article