Tuberc Respir Dis.
2006 Nov;61(5):447-455. 10.4046/trd.2006.61.5.447.
The Expression of MMPs and TIMPs in IPF and NSIP
- Affiliations
-
- 1Division of Pulmonary Medicine, Department of Internal Medicine, Gachon Medical School Gil Medical Center, Incheon, Korea. jsw@gilhospital.com
- 2Department of Pathology, Gachon Medical School Gil Medical Center, Incheon, Korea.
- KMID: 1970250
- DOI: http://doi.org/10.4046/trd.2006.61.5.447
Abstract
-
BACKGROUND: MMPs and TIMPs are important factors for abnormal remodeling the pulmonary parenchyme in idiopathic interstitial pneumonia(IIP) This study evaluated the expression of MMPs and TIMPs in the tissue of IPF, NSIP and normal control subjects.
METHOD: The MMP-2 and -9 activity in the lung tissue was studied by gelatin zymography, and the expression of MMP-1, -2 ,-9, TIMP-1 and -2 in the lung tissue was measured by immunohistochemistry. Thirty five patients, who were diagnosed with IIP (UIP ; 22, NSIP ; 13), were enrolled in the immunohistochemical study. Thirteen patients with IIP (UIP ; 9, NSIP ; 4) and five patients with lung cancer were enrolled in the zymographic assay.
RESULTS
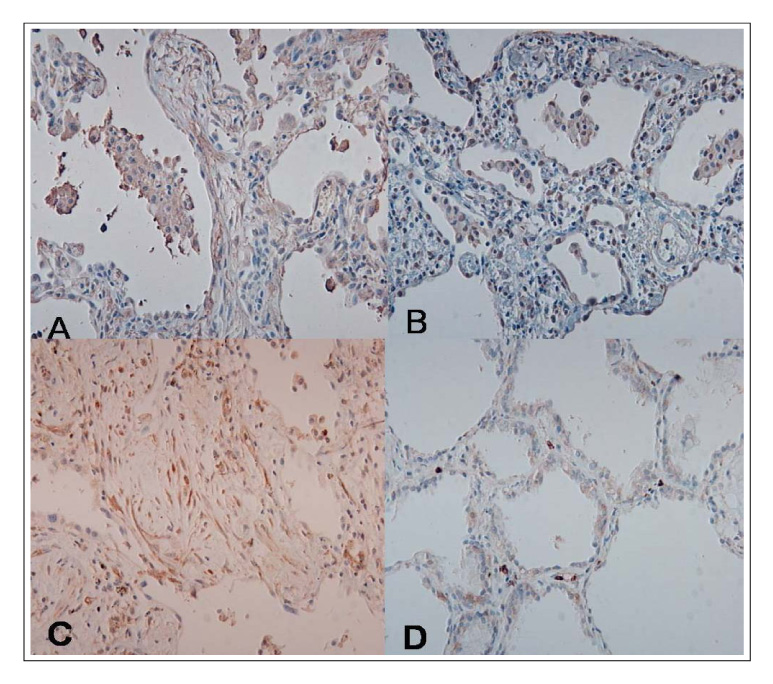
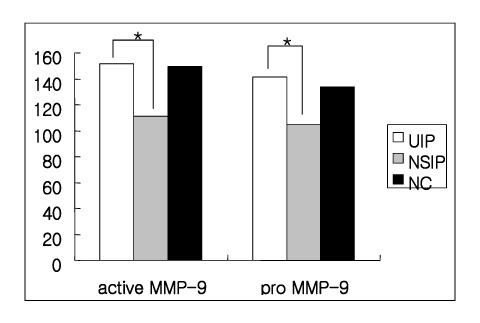
(1) The immunohistochemistry for MMP-1,-2,-9, TIMP-1 and-2 ; MMP-1,-9 and TIMP-2 were stained stronger in the UIP subjects than NSIP and the normal control. TIMP-2 was strongly stained in the UIP tissue. particularly the fibroblasts in the fibroblastic foci. (2) Zymography for MMP-2 and MMP-9 revealed MMP-2 to have prominent expression in the UIP tissue than in the NSIP tissue.
CONCLUSIONS
These results suggest that the overexpression of the TIMPs and gelatinases in UIP might be? important factors in the irreversible fibrosis of the lung parenchyme.
MeSH Terms
-
Fibroblasts
Fibrosis
Gelatin
Gelatinases
Humans
Immunohistochemistry
Lung
Lung Neoplasms
Matrix Metalloproteinases*
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Gelatin
Gelatinases
Matrix Metalloproteinases
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Figure
Reference
-
1. American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002. 165:277–304.2. Gross TJ, Hunninhake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001. 345:517–525.3. Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis: histologic features and clinical significance. Am J Surg Pathol. 1994. 18:136–147.4. Leslie KO. Historical perspictive: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest. 2005. 128:513S–519S.5. Kim DS. An CM, editor. idiopathic interstitial pneumonia. Respiratory diseases. 2004. Seoul: Koonja publishing Inc;457–467.6. Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, et al. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol. 1996. 149:1241–1256.7. Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992. 7:120–125.8. Selman M, Ruiz V, Cabrea S, Segura L, Ramirez R, Barrios R, et al. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis: aprevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000. 279:L562–L574.9. Fukuda Y, Ishizake M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9, and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab invest. 1998. 78:687–698.10. Madtes DK, Eliston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001. 24:599–607.11. Choi KH, Lee HB, Jeong MY, Rhee YK, Chung MJ, Kwak YG, et al. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in cryptogenic organizing pneumonia. Chest. 2002. 121:1478–1485.12. Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, et al. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2000. 162:1949–1956.13. Park JH, Shim TS, Lim CM, Koh YS, Lee SD, Kim WS, et al. Matrix metalloproteinases in idiopathic pulmonary fibrosis. Tuberc Respir Dis. 2001. 51:303–314.14. Kyung SY, Lim YH, An CH, Park JW, Jeong SH, Shin EK, et al. Immunohistochemical study of metalloproteinase-1 and tissue inhibitor of matrix metalloproteinase-1, -2 in idiopathic interstitial pneumonia. Korean J Med. 2003. 65:196–204.15. Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications fortherapy. Ann Intern Med. 2001. 134:136–151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical study of metalloproteinase-1 and tissue inhibitor of matrix metalloproteinase-1, -2 in the idiopathic interstitial pneumonia
- Role of Matrix Metalloproteinase-9 in Asthma
- Differential Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Thioacetamide-Induced Chronic Liver Injury
- The Expression of Matrix Metalloproteinase according to Hydrostatic Pressure in Varicose Veins
- Expression of Matrix Metalloproteinases and Its Inhibitor in Gastric Adenocarcinoma