Porcine epidemic diarrhea: a review of current epidemiology and available vaccines
- Affiliations
-
- 1Department of Pharmacy, College of Pharmacy, Korea University, Sejong, Korea.
- 2Research Unit, Green Cross Veterinary Products, Yongin, Korea. suyun@gcvp.co.kr
- KMID: 1965401
- DOI: http://doi.org/10.7774/cevr.2015.4.2.166
Abstract
- Porcine epidemic diarrhea virus (PEDV), an Alphacoronavirus in the family Coronaviridae, causes acute diarrhea, vomiting, dehydration, and high mortality rates in neonatal piglets. PEDV can also cause diarrhea, agalactia, and abnormal reproductive cycles in pregnant sows. Although PEDV was first identified in Europe, it has resulted in significant economic losses in many Asian swine-raising countries, including Korea, China, Japan, Vietnam, and the Philippines. However, from April 2013 to the present, major outbreaks of PEDV have been reported in the United States, Canada, and Mexico. Moreover, intercontinental transmission of PEDV has increased mortality rates in seronegative neonatal piglets, resulting in 10% loss of the US pig population. The emergence and re-emergence of PEDV indicates that the virus is able to evade current vaccine strategies. Continuous emergence of multiple mutant strains from several regions has aggravated porcine epidemic diarrhea endemic conditions and highlighted the need for new vaccines based on the current circulating PEDV. Epidemic PEDV strains tend to be more pathogenic and cause increased death in pigs, thereby causing substantial financial losses for swine producers. In this review, we described the epidemiology of PEDV in several countries and present molecular characterization of current strains. We also discuss PEDV vaccines and related issues.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Isolation and characterization of a new porcine epidemic diarrhea virus variant that occurred in Korea in 2014
Dong-Kun Yang, Ha-Hyun Kim, Seung-Heon Lee, Soon-Seek Yoon, Jung-Won Park, In-Soo Cho
J Vet Sci. 2018;19(1):71-78. doi: 10.4142/jvs.2018.19.1.71.Enhancement of antigen-specific humoral immune responses and protein solubility through conjugation of bacterial flagellin, Vibrio vulnificus FlaB, to the N-terminus of porcine epidemic diarrhea virus surface protein antigen S0
Seo-ho Oh, Young-Saeng Kim Cho, Ho-Bin Lee, Sang-Mok Lee, Whee-Soo Kim, Liang Hong, Chong-Su Cho, Yun-Jaie Choi, Sang-Kee Kang
J Vet Sci. 2019;20(6):. doi: 10.4142/jvs.2019.20.e70.Efficacy of inactivated variant porcine epidemic diarrhea virus vaccines in growing pigs
Seung Heon Lee, Dong-Kun Yang, Ha-Hyun Kim, In-Soo Cho
Clin Exp Vaccine Res. 2018;7(1):61-69. doi: 10.7774/cevr.2018.7.1.61.
Reference
-
1. Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978; 58:243–247.
Article2. Shibata I, Tsuda T, Mori M, Ono M, Sueyoshi M, Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet Microbiol. 2000; 72:173–182.
Article3. Sun RQ, Cai RJ, Chen YQ, Liang PS, Chen DK, Song CX. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis. 2012; 18:161–163.
Article4. Oldham J. Letter to the editor. Pig Farming. 1972; 10:72–73.
Article5. Jinghui F, Yijing L. Cloning and sequence analysis of the M gene of porcine epidemic diarrhea virus LJB/03. Virus Genes. 2005; 30:69–73.
Article6. Kusanagi K, Kuwahara H, Katoh T, et al. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. J Vet Med Sci. 1992; 54:313–318.
Article7. Song D, Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012; 44:167–175.
Article8. Kweon CH, Kwon BJ, Jung TS, et al. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J Vet Res. 1993; 33:249–254.9. Takahashi K, Okada K, Ohshima K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. J Vet Med Sci. 1983; 45:829–832.
Article10. Vlasova AN, Marthaler D, Wang Q, et al. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg Infect Dis. 2014; 20:1620–1628.
Article11. Cho YY, Lim SI, Kim YK, Song JY, Lee JB, An DJ. Complete genome sequence of K14JB01, a novel variant strain of porcine epidemic diarrhea virus in South Korea. Genome Announc. 2014; 2:e00505–e00514.
Article12. Lin CN, Chung WB, Chang SW, et al. US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013-2014. J Vet Med Sci. 2014; 76:1297–1299.
Article13. Huang YW, Dickerman AW, Pineyro P, et al. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio. 2013; 4:e00737–e00713.
Article14. PED vaccine gains conditional approval. J Am Vet Med Assoc. 2014; 245:267.15. ESFA Panel on Animal Health and Welfare (AHAW). Scientific opinion on porcine epidemic diarrhoea and emerging porcine deltacoronavirus. EFSA J. 2014; 12:3877.16. Sun R, Leng Z, Zhai SL, Chen D, Song C. Genetic variability and phylogeny of current Chinese porcine epidemic diarrhea virus strains based on spike, ORF3, and membrane genes. ScientificWorldJournal. 2014; 2014:208439.17. Jung K, Saif LJ. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J. 2015; 204:134–143.
Article18. Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003; 77:8801–8811.
Article19. Cruz DJ, Kim CJ, Shin HJ. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize porcine epidemic diarrhea virus. Virus Res. 2008; 132:192–196.
Article20. Chang SH, Bae JL, Kang TJ, et al. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells. 2002; 14:295–299.21. Godet M, Grosclaude J, Delmas B, Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J Virol. 1994; 68:8008–8016.
Article22. Park SJ, Moon HJ, Yang JS, et al. Sequence analysis of the partial spike glycoprotein gene of porcine epidemic diarrhea viruses isolated in Korea. Virus Genes. 2007; 35:321–332.
Article23. Park SJ, Moon HJ, Luo Y, et al. Cloning and further sequence analysis of the ORF3 gene of wild- and attenuated-type porcine epidemic diarrhea viruses. Virus Genes. 2008; 36:95–104.
Article24. Chen JF, Sun DB, Wang CB, et al. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes. 2008; 36:355–364.
Article25. Chen J, Wang C, Shi H, et al. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch Virol. 2010; 155:1471–1476.
Article26. Chen Q, Li G, Stasko J, et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014; 52:234–243.
Article27. Meng F, Ren Y, Suo S, et al. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS One. 2013; 8:e57468.
Article28. Luo Y, Zhang J, Deng X, Ye Y, Liao M, Fan H. Complete genome sequence of a highly prevalent isolate of porcine epidemic diarrhea virus in South China. J Virol. 2012; 86:9551.
Article29. Li W, Li H, Liu Y, et al. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012; 18:1350–1353.
Article30. Park SJ, Song DS, Park BK. Molecular epidemiology and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field isolates in Korea. Arch Virol. 2013; 158:1533–1541.
Article31. Correa I, Jimenez G, Sune C, Bullido MJ, Enjuanes L. Antigenic structure of the E2 glycoprotein from transmissible gastroenteritis coronavirus. Virus Res. 1988; 10:77–93.
Article32. Delmas B, Rasschaert D, Godet M, Gelfi J, Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J Gen Virol. 1990; 71(Pt 6):1313–1323.
Article33. Hao J, Xue C, He L, Wang Y, Cao Y. Bioinformatics insight into the spike glycoprotein gene of field porcine epidemic diarrhea strains during 2011-2013 in Guangdong, China. Virus Genes. 2014; 49:58–67.
Article34. Park S, Kim S, Song D, Park B. Novel porcine epidemic diarrhea virus variant with large genomic deletion, South Korea. Emerg Infect Dis. 2014; 20:2089–2092.
Article35. Oka T, Saif LJ, Marthaler D, et al. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet Microbiol. 2014; 173:258–269.
Article36. Ruch TR, Machamer CE. The coronavirus E protein: assembly and beyond. Viruses. 2012; 4:363–382.
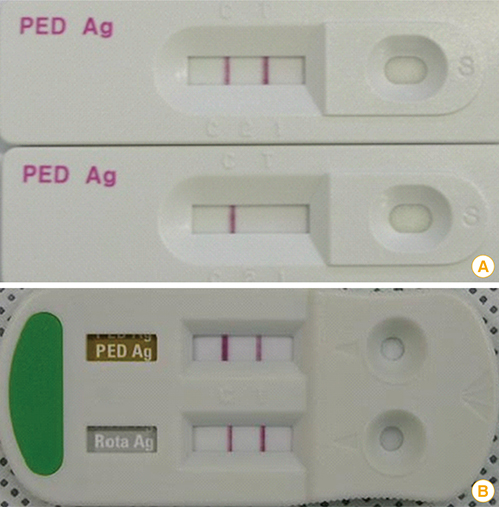
Article37. Rodak L, Valicek L, Smid B, Nevorankova Z. An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. Vet Microbiol. 2005; 105:9–17.
Article38. Martelli P, Lavazza A, Nigrelli AD, Merialdi G, Alborali LG, Pensaert MB. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet Rec. 2008; 162:307–310.
Article39. Sozzi E, Luppi A, Lelli D, et al. Comparison of enzyme-linked immunosorbent assay and RT-PCR for the detection of porcine epidemic diarrhoea virus. Res Vet Sci. 2010; 88:166–168.
Article40. Sozzi E, Papetti A, Lelli D, et al. Diagnosis and investigations on PED in Northern Italy. In : The 8th Annual EPIZONE Meeting; 2014 Sep 23-25; Copenhagen, Denmark.41. Temeeyasen G, Srijangwad A, Tripipat T, et al. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect Genet Evol. 2014; 21:205–213.
Article42. Puranaveja S, Poolperm P, Lertwatcharasarakul P, et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg Infect Dis. 2009; 15:1112–1115.
Article43. Olanratmanee EO, Kunavongkrit A, Tummaruk P. Impact of porcine epidemic diarrhea virus infection at different periods of pregnancy on subsequent reproductive performance in gilts and sows. Anim Reprod Sci. 2010; 122:42–51.
Article44. Ayudhya SN, Assavacheep P, Thanawongnuwech R. One world--one health: the threat of emerging swine diseases. An Asian perspective. Transbound Emerg Dis. 2012; 59:Suppl 1. 9–17.45. Morales RG, Umandal AC, Lantican CA. Emerging and re-emerging diseases in Asia and the Pacific with special emphasis on porcine epidemic diarrhoea. Conf OIE. 2008; 2007:185–189.46. Lee DK, Park CK, Kim SH, Lee C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Res. 2010; 149:175–182.
Article47. Duy DT, Toan NT, Puranaveja S, Thanawongnuwech R. Genetic characterization of porcine epidemic diarrhea virus (PEDV) isolates from southern Vietnam during 2009-2010 outbreaks. Thai J Vet Med. 2011; 41:55–64.48. Wang H, Xia X, Liu Z, et al. Outbreak of porcine epidemic diarrhea in piglets in Gansu province, China. Acta Sci Vet. 2013; 41:1140.49. Feng L. The updated epidemic and controls of swine enteric coronavirus in China. In : International Conference on Swine Enteric Coronavirus Diseases; 2014 Sep 23-25; Chicago, IL, USA.50. Lee S, Lee C. Outbreak-related porcine epidemic diarrhea virus strains similar to US strains, South Korea, 2013. Emerg Infect Dis. 2014; 20:1223–1226.
Article51. Choi JC, Lee KK, Pi JH, et al. Comparative genome analysis and molecular epidemiology of the reemerging porcine epidemic diarrhea virus strains isolated in Korea. Infect Genet Evol. 2014; 26:348–351.
Article52. Fajardo R, Alpizar A, Martinez A, et al. Two cases report of PED in different states in México. In : Proceedings of the 23rd International Pig Veterinary Society (IPVS) Congress; 2014 Jun 8-11; Cancun, Mexico.53. Quevedo-Valle MV. Porcine epidemic diarrhea outbreak in Peru. In : International Conference on Swine Enteric Coronavirus Diseases; 2014 Sep 23-25; Chicago, IL, USA.54. Gomez L. Diarrea epidémica porcina (DEP) en Republica Dominicana. In : International Conference on Swine Enteric Coronavirus Diseases; 2014 Sep 23-25; Chicago, IL, USA.55. Rativa N. Situacion de la diarrea epidemica porcina en Colombia. In : International Conference on Swine Enteric Coronavirus Diseases; 2014 Sep 23-25; Chicago, IL, USA.56. Ishikawa K, Sekiguchi H, Ogino T, Suzuki S. Direct and rapid detection of porcine epidemic diarrhea virus by RT-PCR. J Virol Methods. 1997; 69:191–195.
Article57. Kweon CH, Lee JG, Han MG, Kang YB. Rapid diagnosis of porcine epidemic diarrhea virus infection by polymerase chain reaction. J Vet Med Sci. 1997; 59:231–232.
Article58. Tobler K, Ackermann M. PEDV leader sequence and junction sites. Adv Exp Med Biol. 1995; 380:541–542.
Article59. Tobler K, Ackermann M. Identification and characterization of new and unknown coronaviruses using RT-PCR and degenerate primers. Schweiz Arch Tierheilkd. 1996; 138:80–86.60. Kim SY, Song DS, Park BK. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J Vet Diagn Invest. 2001; 13:516–520.
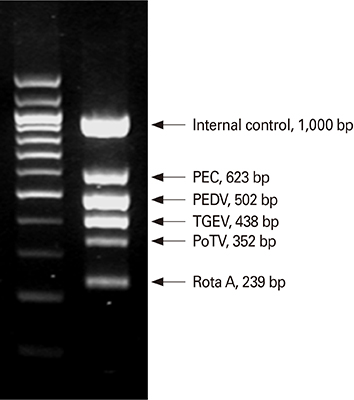
Article61. Song DS, Oh JS, Kang BK, et al. Fecal shedding of a highly cell-culture-adapted porcine epidemic diarrhea virus after oral inoculation in pigs. J Swine Health Prod. 2005; 13:269–272.62. Song DS, Kang BK, Oh JS, et al. Multiplex reverse transcription-PCR for rapid differential detection of porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine group A rotavirus. J Vet Diagn Invest. 2006; 18:278–281.
Article63. Lee CS, Kang BK, Lee DH, et al. One-step multiplex RT-PCR for detection and subtyping of swine influenza H1, H3, N1, N2 viruses in clinical samples using a dual priming oligonucleotide (DPO) system. J Virol Methods. 2008; 151:30–34.
Article64. Ren X, Suo S, Jang YS. Development of a porcine epidemic diarrhea virus M protein-based ELISA for virus detection. Biotechnol Lett. 2011; 33:215–220.
Article65. Ren X, Li P. Development of reverse transcription loop-mediated isothermal amplification for rapid detection of porcine epidemic diarrhea virus. Virus Genes. 2011; 42:229–235.
Article66. Saif LJ. Enteric viral infections of pigs and strategies for induction of mucosal immunity. Adv Vet Med. 1999; 41:429–446.
Article67. Offit PA, Clark HF. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985; 54:58–64.
Article68. Bohl EH, Gupta RK, Olquin MV, Saif LJ. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972; 6:289–301.
Article69. Saif LJ, Bohl EH, Gupta RK. Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect Immun. 1972; 6:600–609.
Article70. Schwartz K, Henry S, Tokach L, Potter M, Davidson D, Egnor C. Exposing sows to PEDV to build herd immunity [Internet]. Minneapolis, MN: National Hog Farmer;2014. cited 2014 Mar 13. Available from: http://nationalhogfarmer.com/business/exposing-sows-pedv-build-herd-immunity.71. Park JS, Ha Y, Kwon B, Cho KD, Lee BH, Chae C. Detection of porcine circovirus 2 in mammary and other tissues from experimentally infected sows. J Comp Pathol. 2009; 140:208–211.
Article72. Ha Y, Shin JH, Chae C. Colostral transmission of porcine circovirus 2 (PCV-2): reproduction of post-weaning multisystemic wasting syndrome in pigs fed milk from PCV-2-infected sows with post-natal porcine parvovirus infection or immunostimulation. J Gen Virol. 2010; 91:1601–1608.
Article73. Song DS, Oh JS, Kang BK, et al. Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res Vet Sci. 2007; 82:134–140.
Article74. de Arriba ML, Carvajal A, Pozo J, Rubio P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhoea virus. Vet Immunol Immunopathol. 2002; 85:85–97.
Article75. Pan Y, Tian X, Li W, et al. Isolation and characterization of a variant porcine epidemic diarrhea virus in China. Virol J. 2012; 9:195.
Article76. Tian Y, Yu Z, Cheng K, et al. Molecular characterization and phylogenetic analysis of new variants of the porcine epidemic diarrhea virus in Gansu, China in 2012. Viruses. 2013; 5:1991–2004.
Article77. Kim SH, Lee JM, Jung J, et al. Genetic characterization of porcine epidemic diarrhea virus in Korea from 1998 to 2013. Arch Virol. 2015; 160:1055–1064.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent vaccine technology in industrial animals
- Genetic diversity of nucleocapsid genes of recent porcine epidemic diarrhea viruses isolated in Korea
- Porcine epidemic diarrhea virus: an update overview of virus epidemiology, vaccines, and control strategies in South Korea
- Reemergence of porcine epidemic diarrhea virus on Jeju Island
- Sequence analysis of the spike gene of Porcine epidemic diarrhea virus isolated from South China during 2011–2015