J Korean Med Assoc.
2005 Apr;48(4):377-384. 10.5124/jkma.2005.48.4.377.
Hormone Therapy in Postmenopausal Women
- Affiliations
-
- 1Department of Interanl Medicine, Sungkyunkwan University of Medicine, Samsung Cheil Hospital and Woman's Healthcare Center, Korea. hankiok@medigate.net
- KMID: 1905315
- DOI: http://doi.org/10.5124/jkma.2005.48.4.377
Abstract
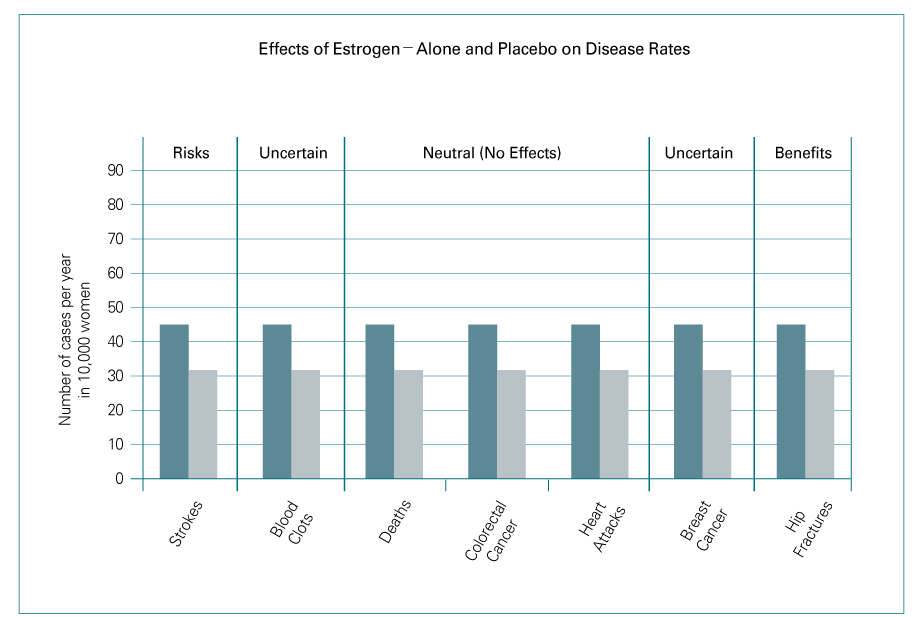
- Until the results of Women's Health Initiative (WHI) was released in July 2002, hormone replacement after menopause had been thought to be the most effective way to manage menopause-related symptoms and to prevent longterm related diseases including osteoporosis, cardiovascular disease, and Alzheimer's disease. A significant increase in breast cancer incidence (by 26%) brought the early termination of the WHI study. After an assessment of the overall risk-benefit ratio, the WHI investigators failed to demonstrate beneficial effects of the combined hormone therapy. This article reviews the results of several large randomized controlled studies and discusses the risks and benefits of hormone therapy.
MeSH Terms
Figure
Reference
-
1. Hulley S, Grady G, Bush T, Furberg C, Herrington D, Vittinghoff E, et al. Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. JAMA. 1998. 280:605–613.
Article2. Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Wenger N, et al. Cardiovascular Disease Outcomes During 6.8 Years of Hormone Therapy Heart and EstrogenProgestin Replacement Study Follow-. JAMA. 2002. 288:49–57.
Article3. Writing Group for the Women's Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women Principal Results From the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002. 288:321–333.4. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Cushman M, et al. Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003. 349:523–534.
Article5. Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, McTiernan A, et al. WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003. 289:3243–3253.
Article6. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, Watts NB, et al. Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003. 290:1729–1738.
Article7. Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Mysiw WJ, et al. WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Womens Health Initiative: a randomized trial. JAMA. 2003. 289:2673–2678.
Article8. Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Bowen D, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Womens Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003. 289:2663–2672.
Article9. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Wactawski-Wende J, et al. WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003. 289:2651–2662.
Article10. Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Valanis BG, et al. Women's Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003. 348:1839–1854.
Article11. Garnet LA, Howard LJ, Andrew MK, David HB, Shirley AA, Ana ML, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures: The Women's Health Initiative Randomized Trial. JAMA. 2003. 290:1739–1748.
Article12. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004. 291:1701–1712.13. Linsay R, Hart OM, Clark OM. The minimum effective dose of estrogen for the prevention of postmenopausal bone loss. Obstet Gynecol. 1984. 63:759–763.14. Linsay R, Gallagher JC, Kleerekoper M, Kleerekoper M, Pickar JH. Effects of lower doses of conjugated equine estrogens with and without me-roxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002. 287:2668–2676.
Article15. Utian WH, Shoupe D, Bachman G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with low doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001. 75:1065–1079.
Article16. Prestwood KM, Thompson DL, Kenny AM, Seibel MJ, Pilbeam CC, Raisz LG. Low dose estrogen and calcium have an additive effect on bone resorption in older women. J Clin Endocrinol Metab. 1999. 84:179–183.
Article17. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultra-low-dose micronized 17 beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003. 290:1042–1048.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Hormone Replacement upon hs- CRP in Korean Postmenopausal Women
- Breast Parenchymal Change on Mammography Following Postmenopausal Hormone Replacement Therapy
- The usefulness of laparoscopic myomectomy after Hormone Replacement Therapy in postmenopausal women with uterine myoma
- A Survey of Obstetricians & Gynecologists' Attitudes on Hormone Replacement Therapy
- Hormone Replacement Therapy for Postmenopausal Women