Korean J Urol.
2007 Jun;48(6):646-651. 10.4111/kju.2007.48.6.646.
The Refill Rate and Reasons for Abandonment of Phosphodiesterase5 Inhibitors in Erectile Dysfunction Patients
- Affiliations
-
- 1Department of Urology, Sungkyunkwan University College of Medicine, Seoul, Korea. drsw.lee@samsung.com
- KMID: 1885548
- DOI: http://doi.org/10.4111/kju.2007.48.6.646
Abstract
-
PURPOSE: The aim of this study was to determine the rate of abandonment of phosphodiesterase (PDE)5 inhibitor therapy in patients who had reported good treatment efficacy, and to assess the reasons for abandonment of therapy and rate of prescription refilling.
MATERIALS AND METHODS
Between January 2004 and December 2005, patients with erectile dysfunction (ED) who had begun PDE5 inhibitor therapy in our center were enrolled in this study. A telephone survey of these patients was conducted to determine the rate of prescription refilling and the reasons for abandonment. After receiving the first prescription, patients were asked whether they had used the medication and whether the treatment improved their erectile function using a Global Assessment Questionnaire (GAQ). Medical records for these patients were reviewed retrospectively.
RESULTS
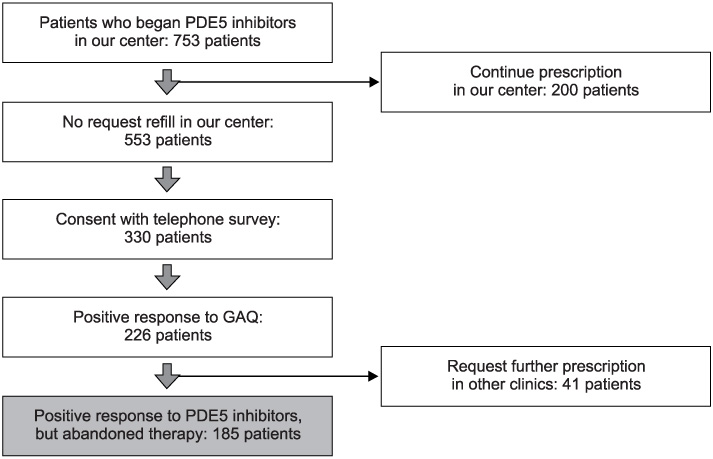
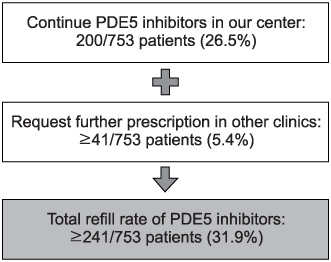
753 patients with ED began PDE5 inhibitor therapy. Only 200 subjects (26.5%) asked for a refill of PDE5 inhibitors. Of 553 subjects, 330 consented to the telephone survey and 226 subjects (68.5%) had positive response on the GAQ. Forty-one patients continued taking PDE5 inhibitors in other clinics. Finally, the reasons for abandonment were assessed in 185 patients who had reported good treatment efficacy but abandoned therapy. The majority reported that they had experienced improvement of spontaneous erectile function (21.6%). Two hundred subjects requested refills in our center (26.5%) and 41 patients (5.4%) continued a prescription in other clinics, so the rate of refill was greater than 31.9%.
CONCLUSIONS
According to the results of this study, the improvement of ED was cited as the major reason for abandonment of PDE5 inhibitor therapy. The rate of prescription refilling was greater than 31.9%.
MeSH Terms
Figure
Cited by 1 articles
-
The Prescribing and Dispensing of Phosphodiesterase Type 5 Inhibitors in South Korea: A Questionnaire Survey of Patient Discomfort
Sang Woo Kim, Jong Wook Kim, Ji Yun Chae, Jin Wook Kim, Cheol Yong Yoon, Mi Mi Oh, Hong Seok Park, Je Jong Kim, Du Geon Moon
World J Mens Health. 2014;32(2):69-75. doi: 10.5534/wjmh.2014.32.2.69.
Reference
-
1. NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993. 270:83–90.2. Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the 'Cologne Male Survey'. Int J Impot Res. 2000. 12:305–311.3. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, Mc-Kinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994. 151:54–61.4. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999. 84:50–56.5. Kim NN. Phosphodiesterase type 5 inhibitors: a biochemical and clinical correlation survey. Int J Impot Res. 2003. 15:Suppl 5. S13–S19.6. Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003. 92:9M–18M.7. Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The Multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004. 20:607–617.8. Klotz T, Mathers M, Klotz R, Sommer F. Why do patients with erectile dysfunction abandon effective therapy with sildenafil (Viagra)? Int J Impot Res. 2005. 17:2–4.9. Kim MS, Myung SC, Kim SC. Perceptions of erectile dysfunction treatment of patients visiting a university hospital in the era of multiple oral agents. Korean J Androl. 2006. 24:18–22.10. Son HC, Shin JW, Lim DJ, Park KJ, Kim SW, Paick JS. Sildenafil compliance after restoration of erectile function. Korean J Androl. 2004. 22:11–13.11. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999. 281:537–544.12. Braun M, Klotz T, Mathers MJ, Klingebiel J, Zumbe J, Schoenenberger A, et al. 'Viagra effect' - influence of mass media on patient behavior. Urol Int. 2001. 66:145–148.13. Christiansen E, Guirguis WR, Cox D, Osterloh IH. Long-term efficacy and safety of oral Viagra (sildenafil citrate) in men with erectile dysfunction and the effect of randomised treatment withdrawal. Int J Impot Res. 2000. 12:177–182.14. van Lankveld JJ, van den Hout MA, Spigt MG, van Koeveringe GA. Cognitive changes predict continued recovery of erectile functioning versus relapse after discontinuation of sildenafil treatment for male erectile dysfunction. Psychosom Med. 2003. 65:709–718.15. Sommer F, Klotz T, Engelmann U. Improved spontaneous erectile function in men with mild-to-moderate arteriogenic erectile dysfunction treated with a nightly dose of sildenafil for one year: a randomized trial. Asian J Androl. 2007. 9:134–141.16. Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care. 2002. 25:1336–1339.17. Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005. 47:214–220.18. Saenz dTI, Gonzales CN, Heaton J, Hedlund H, Nehra A, Prickard R. Anatomy, physiology and pathophysiology of erectile dysfunction. 2000. Plymouth: Health Publications;65–102.19. Terradas C, Levalle O, Nagelberg A, Mormandi E. Sildenafil improves nocturnal penile erections in organic impotence. Int J Impot Res. 2001. 13:125–129.20. Rochira V, Granata AR, Balestrieri A, Madeo B, Carani C. Effects of sildenafil on nocturnal penile tumescence and rigidity in normal men: randomized, placebo-controlled, crossover study. J Androl. 2002. 23:566–571.21. Park NC, Kim SW, Moon KH, Park KS, Park JK, Shim HB, et al. An open-label, multicenter, flexible dose study to evaluate the efficacy and safety of Viagra (sildenafil citrate) in males with erectile dysfunction and arterial hypertension who are taking antihypertensive treatment. Korean J Urol. 2005. 46:1015–1022.22. Lee U, Lee MH, Kim SY, Ji YH, Hong JH, Ahn TY. Clinical efficacy and safety of sildenafil in the men with erectile dysfunction in Korea. Korean J Urol. 2001. 42:435–440.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The risk factors, diagnosis and treatment guideline of erectile dysfunction
- Chronic Low Dosing of Phosphodiesterase Type 5 Inhibitor for Erectile Dysfunction
- Comparison of the Efficacy, Safety and Patient Preference of the Phosphodiesterase Type 5 Inhibitors for the Patients with Erectile Dysfunction
- Sildenafil Compliance after Restoration of Erectile Function
- Statins and Erectile Dysfunction