Korean J Urol.
2007 Feb;48(2):219-225. 10.4111/kju.2007.48.2.219.
Comparison of the Efficacy, Safety and Patient Preference of the Phosphodiesterase Type 5 Inhibitors for the Patients with Erectile Dysfunction
- Affiliations
-
- 1Department of Urology, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea. tyahn@amc. seoul.kr
- KMID: 2139764
- DOI: http://doi.org/10.4111/kju.2007.48.2.219
Abstract
-
PURPOSE: To compare the clinical efficacy and safety of three phosphodiesterase type 5 (PDE5) inhibitors in the treatment of mele erectile dysfunction according to patient preference.
MATERIALS AND METHODS
Between January 2004 and August 2005, 113 male erectile dysfunctional patients were enrolled to this randomized, prospective, comparative, open-label, triple-crossover study of three PDE5 inhibitors. Patients were assigned to one of six medication schedules, and were prescribed a full dose of the drugs for 8 weeks, with a week of washout period prior to the next drug cycle. The International Index of Erectile Function (IIEF) scores and side effects related with each medication were obtained at the end of study. 48 patients finished all the medications, and completed the study with a global assessment questionnaire on their drug preference and reasons for that preference.
RESULTS
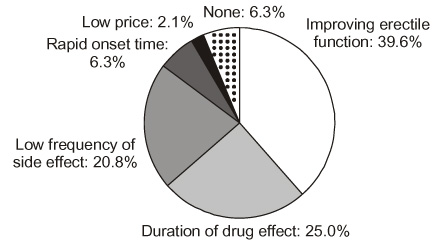
The mean age of the patients was 54.6 (33-73) years. The mean pre-treatment IIEF and EF domain scores (+/-S.D.) were 28.2+/-14.7 and 10.6+/-6.6, respectively. The scores were significantly improved, to 47.9+/-14.6 and 19.9+/-6.6 with sildenafil, to 49.7+/-12.3 and 21.3+/-5.8 with vardenafil, and to 47.9+/-14.9 and 19.8+/-7.2 with tadalafil (p < 0.01). There were no significant differences in the scores or frequencies of side effects between the drugs. The preference percentages were 29.2, 29.2 and 35.4% for sildenafil, vardenafil and tadalafil, respectively. Patient preference was mainly due to improvement in erectile function (70.9%), such as rigid erection, prolonged erection and fast erection, and not to the infrequent rate of side effects (20.8%). CONCLISIONS: There were no significant differences of the efficacy and safety among the three PDE5 inhibitors. The preference for a drug for the treatment of erectile dysfunction was mainly related to the efficacy on the improvement of erectile function rather than the less frequent side effects.
MeSH Terms
Figure
Reference
-
1. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994. 151:54–61.2. Sanchez-Cruz J, Cabrera-Leon A, Martin-Morales A, Fernandez A, Burgos R, Rejas J. Male erectile dysfunction and health-related quality of life. Eur Urol. 2003. 44:245–253.3. Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992. 326:90–94.4. Moore RA, Derry S, McQuay HJ. Indirect comparison of interventions using published randomized trials: systematic review of PDE-5 inhibitors for erectile dysfunction. BMC Urol. 2005. 5:18.5. The process of care model for evaluation and treatment of erectile dysfunction. The Process of Care Consensus Panel. Int J Impot Res. 1999. 11:59.6. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998. 338:1397–1404.7. Omrod D, Easthope SE, Figgitt DP. Vardenafil. Drugs Aging. 2002. 19:217–227.8. Kuan J, Brook G. Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2002. 11:1605–1613.9. Eardley I, Cartledge J. Tadalafil (Cialis®) for men with erectile dysfunction. Int J Clin Pract. 2002. 56:300–304.10. Mulhall JP. Understanding erectile dysfunction medication preference studies. Curr Opin Urol. 2004. 14:367–373.11. Hedelin H, Stroberg P. Treatment for erectile dysfunction based on patient-reported outcomes: to every man the PDE5 inhibitor that he finds superior. Drugs. 2005. 65:2245–2251.12. Govier F, Potempa AJ, Kaufman J, Denne J, Kovalenko P, Ahuja S. A multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20mg or sildenafil citrate 50mg during initiation of treatment for erectile dysfunction. Clin Ther. 2003. 25:2709–2723.13. Stroberg P, Murphy A, Costigan T. Switching patients with erectile dysfunction from sildenafil citrate to tadalafil: results of a European multicenter, open-label study of patient preference. Clin Ther. 2003. 25:2724–2737.14. Mulhall JP, Montorsi F. Evaluating preference trials of oral phosphodiesterase 5 inhibitors for erectile dysfunction. Eur Urol. 2006. 49:30–37.15. Moher D. CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. Consolidated standards of reporting trials. JAMA. 1998. 279:1489–1491.16. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials explanation and elaboration. Ann Intern Med. 2001. 134:663–694.17. Porst H, Arnds S, Kleingarn M. The three PDE 5 inhibitors sildenafil, tadalafil, and vardenafil - results of a comparative preference trial in 222 patients with erectile dysfunction. Eur Urol. 2004. 2:Suppl 3. 408.18. Sommer F, Mathers M, Klotz T, Van Ahlen H, Bondarenko B, Ozgur E, et al. Which PDE5 inhibitor do you prefer? A comparative randomized multicenter study of sildenafil, tadalafil, and vardenafil. J Urol. 2004. 171:Suppl 4. 314–315.19. Claes H, Van Poppel H. The use of sildenafil, tadalafil, and vardenafil in clinical practice. J Sex Med. 2005. 2:Suppl 1. 21.20. Park NC, Park HJ, Nam JK, Kim JM. Efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil, and tadalafil: results of an open label study of patient preference in Korea. J Sex Med. 2004. 1:Suppl 1. 55.21. Yoon CJ, Lee SH, Moon KH, Yoo ES, Park JS, Lee KS, et al. The analysis of preference for three PDE-5 inhibitors. Korean J Androl. 2005. 23:116–121.22. Jackson G, Gillies H, Oterloh I. Past, present, and future: a 7-year update of Viagra® (sildenafil citrate). Int J Clin Pract. 2005. 59:680–691.23. Eardley I, Mirone V, Montorsi F, Ralph D, Kell P, Warner MR, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int. 2005. 96:1323–1332.24. Hatzichristou D, Montorsi F, Buvat J, Larerriere N, Bandel TJ, Porst H, et al. The efficacy and safety of flexible-dose vardenafil (Levitra®) in a broad population of European men. Eur Urol. 2004. 45:634–641.25. Carson CC. PDE5 inhibitors: are there differences? Can J Urol. 2006. 13:Suppl 1. 34–39.26. Montorsi F, Padma-Nathan H, Buvat J, Schwaibold H, Beneke M, Ulbirich E, et al. Earliest time to onset of action leading to successful intercourse with vardenafil determined in an at-home setting: a randomized, double-blind, placebo-controlled trial. J Sex Med. 2004. 1:168–178.27. Shabsigh R, Burnett AL, Eardley J, Sharlip ID, Ellsworth PI, Garcia CS, et al. Time from dosing to sexual intercourse attempts in men taking tadalafil in clinical trials. BJU Int. 2006. 96:857–863.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic Low Dosing of Phosphodiesterase Type 5 Inhibitor for Erectile Dysfunction
- Pharmacokinetics, Efficacy, and Safety of Selective Inhibitors of Phosphodiesterase Type 5 and Sublingual Apomorphine for the Treatment of Erectile Dysfunction
- Treatment Strategy for Non-Responders to PDE5 Inhibitors
- The risk factors, diagnosis and treatment guideline of erectile dysfunction
- The Analysis of Preference for Three PDE-5 Inhibitors