Lab Anim Res.
2011 Sep;27(3):227-234. 10.5625/lar.2011.27.3.227.
In vitro antioxidant properties of a ginseng intestinal metabolite IH-901
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. jkkang@chungbuk.ac.kr
- 2Central Research Institute, Ilhwa Co., Ltd., Guri, Korea.
- 3College of Pharmacy and Medical Research Center, Chungbuk National University, Cheongju, Korea. yjlee1@chungbuk.ac.kr
- KMID: 1850050
- DOI: http://doi.org/10.5625/lar.2011.27.3.227
Abstract
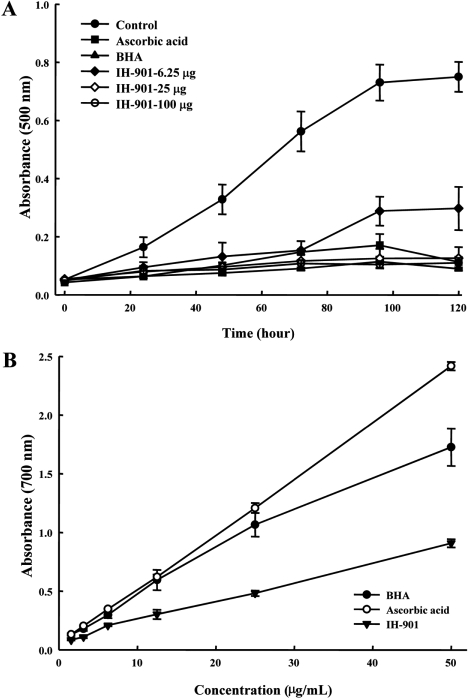
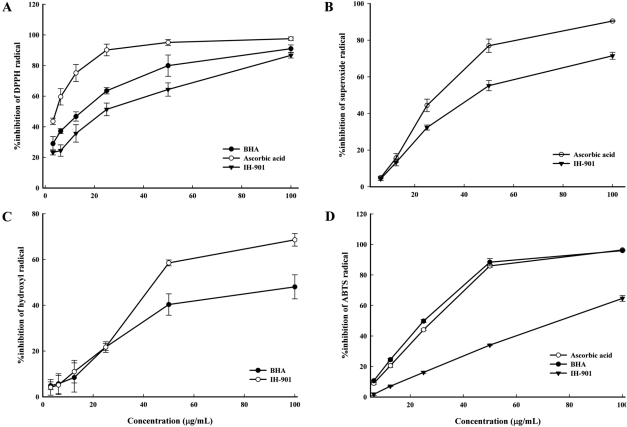
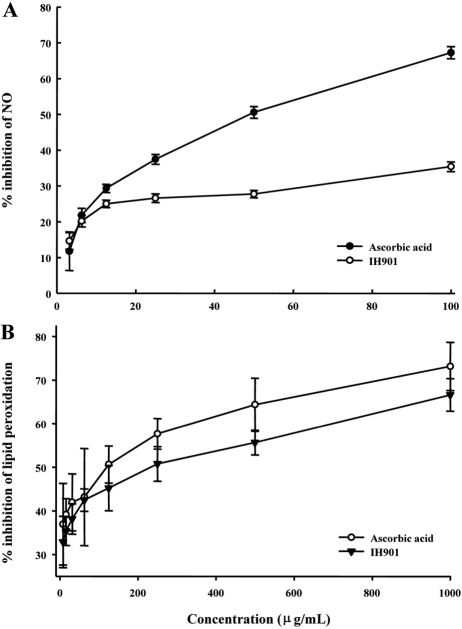
- IH-901 (20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol or compound K) is a final intestinal bacterial metabolite of ginseng in humans. It has various pharmacologic effects such as antiaging, immunopotentiation, antistress, and antimetastatic activities. We analyzed the antioxidant activities of IH-901 using several assays including: total antioxidant activity, reductive potential, 1,1-diphenyl-2-picryl-hydrazyl, hydroxyl, superoxide and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays, a nitric oxide scavenging assay and a lipid peroxidation assay. At concentrations of 25 and 100 microg/mL, IH-901 inhibited lipid peroxidation of a linoleic acid emulsion with a potency comparable to ascorbic acid and butylated hydroxyanisole. The reductive potential of IH-901 increased in a concentration-dependent manner. IH-901 exhibited strong DPPH, hydroxyl, superoxide and ABTS radical scavenging activities. IH-901 was also an effective inhibitor of lipid peroxidation, although IH-901 had only a mild scavenging activity against nitric oxide. These results suggest that IH-901 may be a useful antioxidant agent against reactive oxygen species.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of administration of IH901, a ginsenoside intestinal metabolite, on muscular and pulmonary antioxidant functions after eccentric exercise
Nam-Jin Lee, Jung-Won Lee, Jong-Hwan Sung, Yeoung-Gyu Ko, Seongsoo Hwang, Jong-Koo Kang
J Vet Sci. 2013;14(3):249-256. doi: 10.4142/jvs.2013.14.3.249.
Reference
-
1. Aruoma O. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998; 75(2):199–212.
Article2. Cerutti PA. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991; 21(1):1–5. PMID: 1907547.
Article3. Jung CH, Seog HM, Choi IW, Park MW, Cho HY. Antioxidant properties of various solvent extracts from wild ginseng leaves. Food Sci Technol-LEB. 2006; 39(3):266–274.
Article4. Halliwell B. Antioxidant characterization : Methodology and mechanism. Biochem Pharmacol. 1995; 49(10):1341–1348. PMID: 7763275.5. Jung CH, Seog HM, Choi IW, Cho HY. Antioxidant activities of cultivated and wild Korean ginseng leaves. Food Chem. 2005; 92(3):535–540.
Article6. Kang KS, Yamabe N, Kim HY, Okamoto T, Sei Y, Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007; 113(2):225–232. PMID: 17618072.
Article7. Hasegawa H, Sung JH, Huh JH. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch Pharm Res. 1997; 20(6):539–544. PMID: 18982256.
Article8. Lee BH, Lee SJ, Hui JH, Lee S, Sung JH, Huh JD, Moon CK. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1998; 64(6):500–503. PMID: 9741293.9. Sung JH, Hasegawa H, Matsumiya S, Uchiyama M, Ha JY, Lee MS, Huh JD. Metabolism of ginseng saponins by human intestinal bacteria. Korean J Pharmacogn. 1995; 26:360–367.10. Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996; 20(1):145–150. PMID: 8903691.
Article11. Kang J, Lee Y, No K, Jung E, Sung J, Kim Y, Nam S. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis. Reprod Toxicol. 2002; 16(3):291–298. PMID: 12128103.
Article12. Wang T, Yu X, Qu S, Xu H, Han B, Sui D. Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. Eur J Pharmacol. 2010; 636:121–125. PMID: 20371232.
Article13. Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol. 2004; 56(1):107–113. PMID: 14980007.
Article14. Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. Food Sci Technol-LEB. 2008; 41(6):1060–1066.
Article15. Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986; 44:307–315.16. Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997; 60(10):763–771. PMID: 9064481.
Article17. Aruoma OI. Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol. 1994; 233:57–66.18. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992; 40(6):945–948.
Article19. Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006; 29(9):1906–1910. PMID: 16946507.20. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231–1237. PMID: 10381194.
Article21. Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006; 104(3):322–327. PMID: 16239089.22. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 1986. 2nd ed. Tokyo: Japan Scientific Societies Press;p. 42–53.23. Gülçin I, Küfrevioglu ÖI, Oktay M, Büyüukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004; 90(2-3):205–215. PMID: 15013182.24. Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 2006; 217(2-3):213–220. PMID: 16243424.
Article25. Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984; 219(1):1–14. PMID: 6326753.
Article26. Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem. 2002; 50(23):6882–6890. PMID: 12405792.
Article27. Feng T, Du Y, Li J, Hu Y, Kennedy JF. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr Polym. 2008; 73(1):126–132.
Article28. Razali N, Razab R, Junit SM, Aziz AA. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008; 111(1):38–44.29. Govindarajan R, Rastogi S, Vijayakumar M, Shirwaikar A, Rawat AK, Mehrotra S, Pushpangadan P. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003; 26(10):1424–1427. PMID: 14519948.
Article30. Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J Ethnopharmacol. 2007; 111(3):443–450. PMID: 17276636.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basic Study on the Effect of Korean Ginseng upon Fracture Healing of the Bone
- Influence of Ginseng Powder and Ginseng Saponin on the Growth of Candida albicans in vitro
- Effect of Red Ginseng Extract on Lipid Peroxidation in Streptozotocin-induced Diabetic Rats
- Effect of Ginseng Extract on Blood Lipids and Atherosclerosis
- Brief Introduction of Panax ginseng C.A. Meyer