Korean J Physiol Pharmacol.
2008 Aug;12(4):177-183. 10.4196/kjpp.2008.12.4.177.
Acidic pH-activated Cl- Current and Intracellular Ca2+ Response in Human Keratinocytes
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul, Korea. sjoonkim@snu.ac.kr
- 2Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea.
- 3Laboratory of Cutaneous Aging Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Institute of Dermatological Science, Seoul National University, Seoul, Korea.
- 5Kidney Research Institute, Seoul National University, Seoul, Korea.
- KMID: 1838356
- DOI: http://doi.org/10.4196/kjpp.2008.12.4.177
Abstract
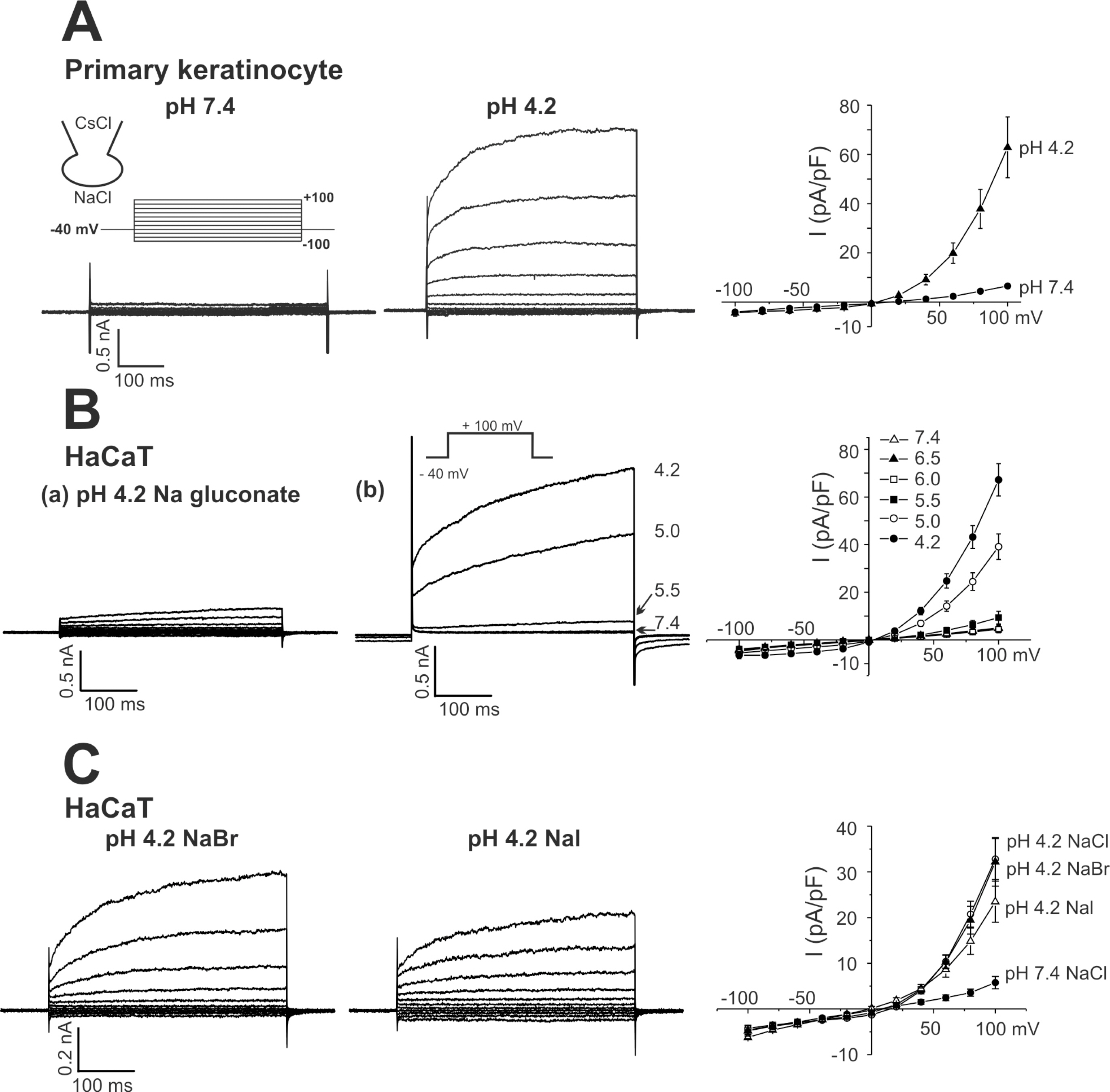
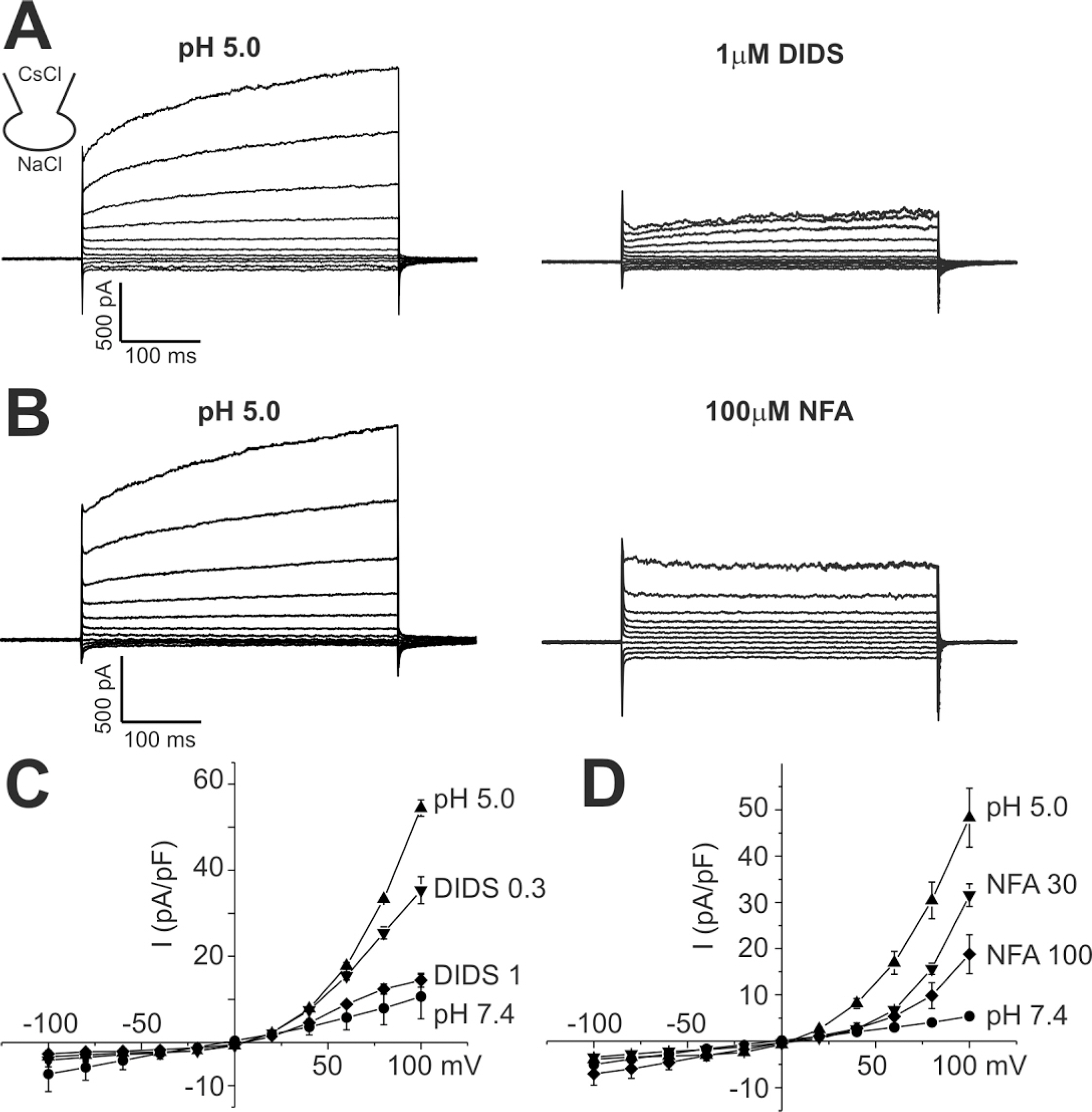
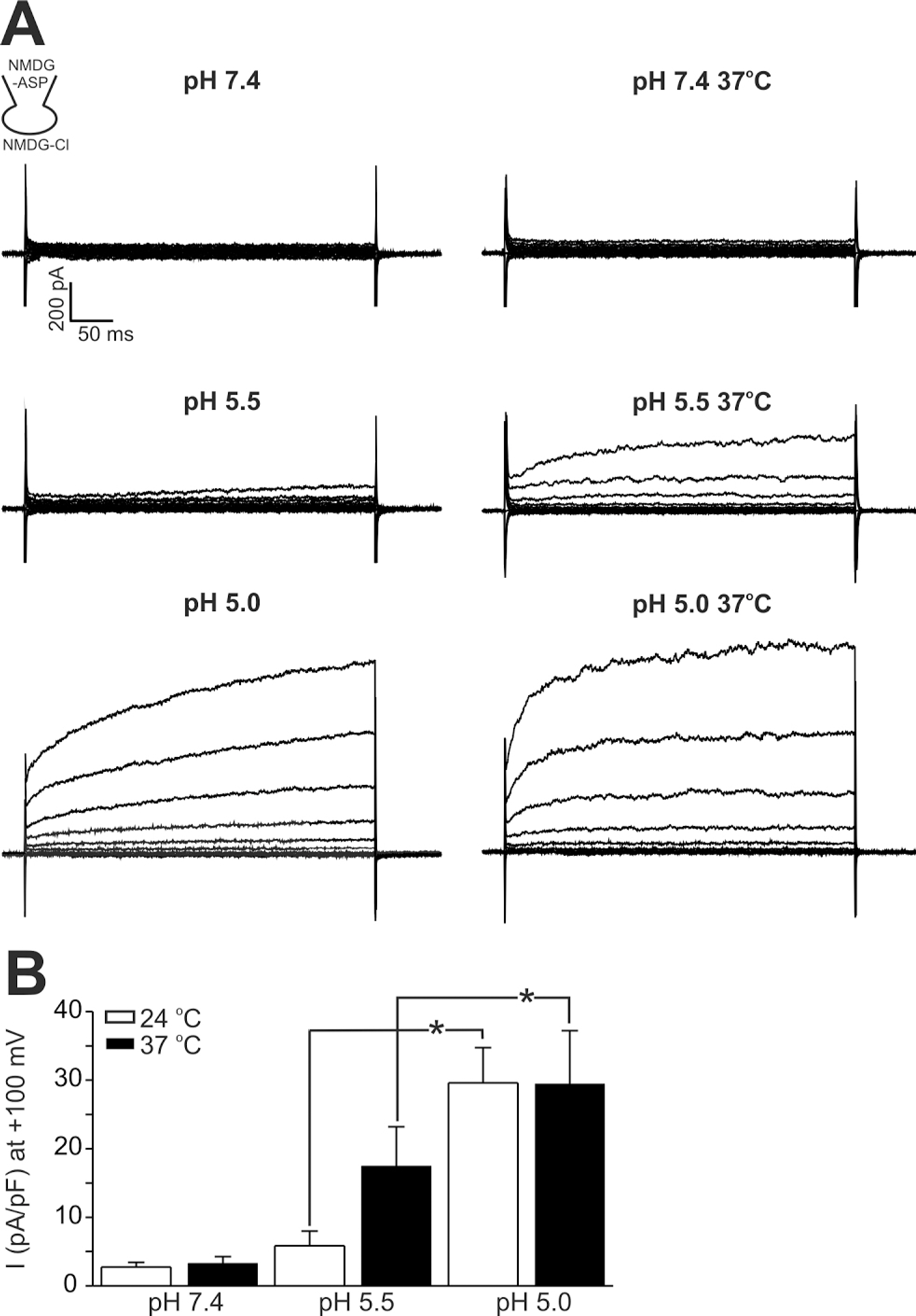
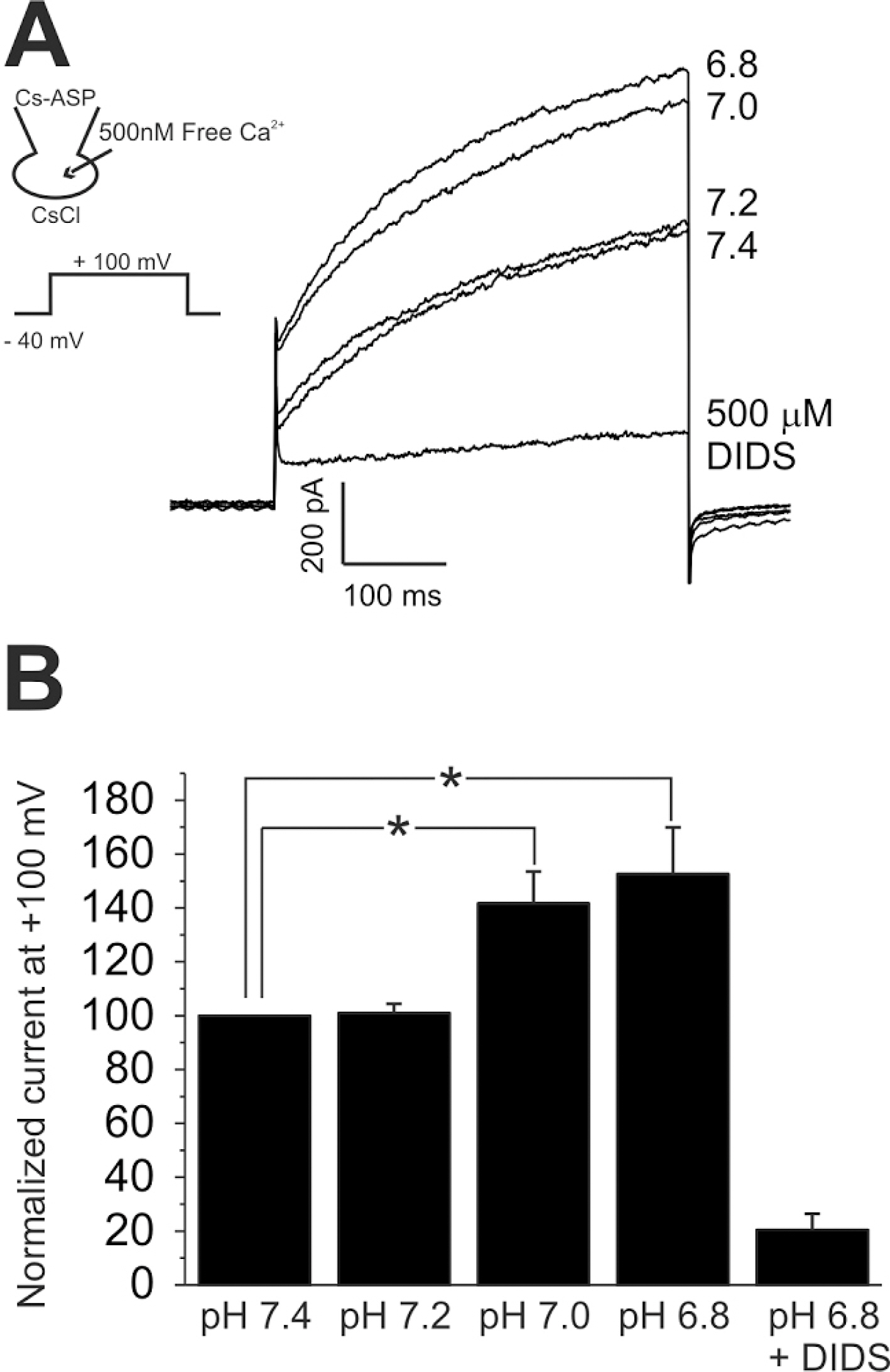
- The layers of keratinocytes form an acid mantle on the surface of the skin. Herein, we investigated the effects of acidic pH on the membrane current and [Ca2+](c) of human primary keratinocytes from foreskins and human keratinocyte cell line (HaCaT). Acidic extracellular pH (pHe< or =5.5) activated outwardly rectifying Cl- current (I(Cl,pH)) with slow kinetics of voltage-dependent activation. I(Cl,pH) was potently inhibited by an anion channel blocker 4,4`-diisothiocyanostilbene-2,2`-disulphonic acid (DIDS, 73.5% inhibition at 1micrometer). I(Cl,pH) became more sensitive to pHe by raising temperature from 24degrees C to 37degrees C. HaCaT cells also expressed Ca2+ -activated Cl- current (I(Cl,Ca)), and the amplitude of I(Cl,Ca) was increased by relatively weak acidic pHe (7.0 and 6.8). Interestingly, the acidic pHe (5.0) also induced a sharp increase in the intracellular [Ca2+] (delta[Ca2+](acid)) of HaCaT cells. The delta[Ca2+](acid) was independent of extracellular Ca2+, and was abolished by the pretreatment with PLC inhibitor, U73122. In primary human keratinocytes, 5 out of 28 tested cells showed delta[Ca2+](acid). In summary, we found I(Cl,pH) and delta[Ca2+](acid) in human keratinocytes, and these ionic signals might have implication in pathophysiological responses and differentiation of epidermal keratinocytes.
Keyword
MeSH Terms
Figure
Reference
-
Auzanneau C., Thoreau V., Kitzis A., Becq F. A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem. 278:19230–19236. 2003.
ArticleBehne MJ., Meyer JW., Hanson KM., Barry NP., Murata S., Crumrine D., Clegg RW., Gratton E., Holleran WM., Elias PM., Mauro TM. NEH1 regulates the stratum corneum permeability barrier homeostasis. J Biol Chem. 277:47299–47406. 2002.Boyce ST., Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 81(1 Suppl):33s–40s. 1983.
ArticleElias PM., Ahn SK., Brown BE., Crumrine D., Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 119:1269–1274. 2002.
ArticleFluhr JW., Kao J., Jain M., Ahn SK., Feingold KR., Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 117:44–51. 2001.
ArticleFluhr JW., Mao-Qiang M., Brown BE., Hachem J-P., Moskowitz DG., Demerjian M., Haftek M., Serre G., Crumrine D., Mauro TM., Elias PM., Feingold KR. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 123:140–151. 2004.
ArticleFrosch P., Kligman AM. Method for appraising the sting capacity of topically applied substances. J Soc Cosmetic Chem. 28:197–209. 1977.Hachem J-P., Crumrine D., Fluhr J., Brown BE., Feingold KR., Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 121:345–353. 2003.
ArticleHirayama Y., Kuruma A., Hiraoka M., Kawano S. Calcium-activated Cl− current is enhanced by acidosis and contributes to the shortening of action potential duration in rabbit ventricular myocytes. Jpn J Physiol. 52:293–300. 2002.Ishii S., Kihara Y., Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 280:9083–9087. 2005.
ArticleKoegel H., Alzheimer C. Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 15:145–154. 2001.Lambert S., Oberwinkler J. Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol. 567:191–213. 2005.
ArticleLang F., Föller M., Lang KS., Lang PA., Ritter M., Gulbins E., Vereninov A., Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Memb Biol. 205:147–157. 2005.
ArticleLehen'kyi V., Beck B., Polakowska R., Charveron M., Bordat P., Skryma R., Prevarskaya N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 282:22582–22591. 2007.Ludwig M-G., Vanek M., Guerini D., Gasser JA., Jones CE., Junker W., Hofstetter H., Wolf RM., Seuwen K. Proton-sensing G-protein coupled receptors. Nature. 425:93–98. 2003.Menkin V. Chemical mediators in relation to cytologic constituents in inflammation. Am J Pathol. 34:921–941. 1958.Menon GK., Elias PM., Lee SH., Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 270:503–512. 1992.
ArticleMurakami N., Yokomizo T., Okuno T., Shimizu T. G2A is a protonsensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 279:42484–42491. 2004.
ArticleNilius B., Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 177:119–147. 2003.
ArticleNobles M., Higgins CF., Sardini A. Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell). Am J Physiol Cell Physiol. 287:C1426–C1435. 2004.Okada Y., Maeno E., Shimizu T., Manabe K., Mori S., Nabekura T. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch. 448:287–295. 2004.
ArticleSilver RB. Ratio imaging: practical considerations for measuring intracellular calcium and pH in living tissue. Methods Cell Biol. 56:237–251. 1998.Smith JB., Dwyer SD., Smith L. Lowering extracellular pH evokes inositol polyphosphate formation and calcium mobilization. J Biol Chem. 264:8723–8728. 1989.
ArticleTu C-L., Chang W., Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 276:41079–41085. 2001.
ArticleWang H-Y., Shimizu T., Numata T., Okada Y. Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch. 454:223–233. 2007.
ArticleWillis CM., Shaw S., Lacharriere ODE., Baverel M., Reiche L., Jourdain R., Bastien P., Wilkinson J. Sensitive skin: an epidemiological study. Br J Dermatol. 145:258–263. 2001.
ArticleYamamoto S., Ehara T. Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am J Physiol Heart Circ Physiol. 290:H1905–H1914. 2006.
ArticleYuspa SH., Kilkenny AE., Steinert PM., Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 109:1207–1217. 1989.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ca2+ signalling in endothelial cells: Role of ion channels
- Intracellular acidosis decreases the outward Na(+)-Ca2+ exchange current in guinea pig ventricular myocytes
- Ca2 -activated K Currents of Pancreatic Duct Cells in Guinea-pig
- Oxidized Low-density Lipoprotein- and Lysophosphatidylcholine-induced Ca2+ Mobilization in Human Endothelial Cells
- Hypoxia Delays the Intracellular Ca2+ Clearance by Na+ - Ca2+ Exchanger in Human Adult Cardiac Myocytes