J Gynecol Oncol.
2012 Oct;23(4):257-264. 10.3802/jgo.2012.23.4.257.
Risk factors for recurrence amongst high intermediate risk patients with endometrioid adenocarcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA. lin@uphs.upenn.edu
- 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
- 3Department of Biostatistics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
- KMID: 1810123
- DOI: http://doi.org/10.3802/jgo.2012.23.4.257
Abstract
OBJECTIVE
To determine risk factors associated with recurrence in patients with high intermediate risk (HIR) endometrioid adenocarcinoma.
METHODS
A retrospective analysis of patients with HIR endometrioid adenocarcinoma who underwent hysterectomy, bilateral salpingo-oophorectomy, with or without pelvic/para-aortic lymphadenectomy at the University of Pennsylvania between 1990 and 2009 was performed.
RESULTS
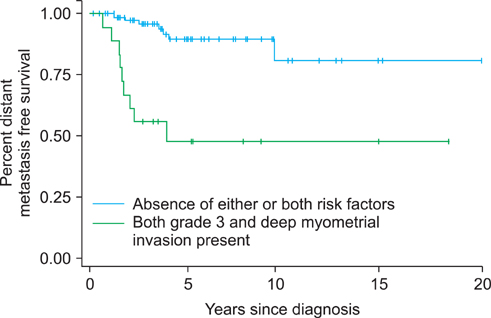
A total of 103 women with HIR endometrial cancer were identified. Multivariable analysis revealed that > or =2/3 myometrial invasion (HR, 4.79; p=0.010) and grade 3 disease (HR, 3.04; p=0.045) were independently predictive of distant metastases. The 5-year distant metastases free survival (DMFS) for patients with neither or one of these risk factors was 89%, and the 5-year DMFS for patients with both risk factors was 48% (p<0.001).
CONCLUSION
Patients with both grade 3 disease and deep third myometrial invasion have a high risk of distant metastases. Identifying these patients may be important in rationally selecting patients for systemic therapy.
MeSH Terms
Figure
Reference
-
1. National Cancer Institute. Endometrial cancer [Internet]. c2012. cited 2012 Aug 20. Bethesda, MD: National Cancer Institute;Available from: http://www.cancer.gov/cancertopics/types/endometrial.2. Lee CM, Szabo A, Shrieve DC, Macdonald OK, Tward JD, Skidmore TB, et al. Descriptive nomograms of adjuvant radiotherapy use and patterns of care analysis for stage I and II endometrial adenocarcinoma: a surveillance, epidemiology, and end results population study. Cancer. 2007. 110:2092–2100.3. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S105–S143.4. Kadar N, Malfetano JH, Homesley HD. Determinants of survival of surgically staged patients with endometrial carcinoma histologically confined to the uterus: implications for therapy. Obstet Gynecol. 1992. 80:655–659.5. Briet JM, Hollema H, Reesink N, Aalders JG, Mourits MJ, ten Hoor KA, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005. 96:799–804.6. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000. 355:1404–1411.7. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004. 92:744–751.8. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991. 40:55–65.9. Ioffe Y, Delic L, Amneus M, Leuchter R, Karlan B, Li A, et al. Before and after GOG 99: did our practice patterns for treatment of intermediate risk endometrial adenocarcinoma change? Gynecol Oncol. 2010. 116:595.10. Rankins NC, Secord AA, Jewell E, Havrilesky LJ, Soper JT, Myers E. Cost-effectiveness of adjuvant radiotherapy in intermediate risk endometrial cancer. Gynecol Oncol. 2007. 106:388–393.11. McMeekin DS, Randall ME. Pelvic radiation therapy or vaginal implant radiation therapy, paclitaxel, and carboplatin in treating patients with high-risk stage I or stage II endometrial cancer. c2012. Bethesda, MD: National Cancer Institute;ClinicalTrials.gov Id: NCT00807768.12. Edge SB, Fritz AG, Byrd DR, Greene FL, Campton CC, Trotti A, editors. Cancer staging handbook: from the AJCC cancer staging manual. 2010. 7th ed. Chicago, IL: American Joint Committee on Cancer.13. Irwin C, Levin W, Fyles A, Pintilie M, Manchul L, Kirkbride P. The role of adjuvant radiotherapy in carcinoma of the endometrium-results in 550 patients with pathologic stage I disease. Gynecol Oncol. 1998. 70:247–254.14. Rush S, Gal D, Potters L, Bosworth J, Lovecchio J. Pelvic control following external beam radiation for surgical stage I endometrial adenocarcinoma. Int J Radiat Oncol Biol Phys. 1995. 33:851–854.15. Gadducci A, Cavazzana A, Cosio S, DI Cristofano C, Tana R, Fanucchi A, et al. Lymph-vascular space involvement and outer one-third myometrial invasion are strong predictors of distant haematogeneous failures in patients with stage I-II endometrioid-type endometrial cancer. Anticancer Res. 2009. 29:1715–1720.16. Rasool N, Fader AN, Seamon L, Neubauer NL, Shahin FA, Alexander HA, et al. Stage I, grade 3 endometrioid adenocarcinoma of the endometrium: an analysis of clinical outcomes and patterns of recurrence. Gynecol Oncol. 2010. 116:10–14.17. Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980. 56:419–427.18. Podczaski E, Kaminski P, Gurski K, MacNeill C, Stryker JA, Singapuri K, et al. Detection and patterns of treatment failure in 300 consecutive cases of "early" endometrial cancer after primary surgery. Gynecol Oncol. 1992. 47:323–327.19. Reddoch JM, Burke TW, Morris M, Tornos C, Levenback C, Gershenson DM. Surveillance for recurrent endometrial carcinoma: development of a follow-up scheme. Gynecol Oncol. 1995. 59:221–225.20. DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in stage I endometrial cancer. Am J Obstet Gynecol. 1985. 151:1009–1015.21. Fanning J, Nanavati PJ, Hilgers RD. Surgical staging and high dose rate brachytherapy for endometrial cancer: limiting external radiotherapy to node-positive tumors. Obstet Gynecol. 1996. 87:1041–1044.22. Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, van den Bergh AC, de Winter KA, Koper PC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004. 22:1234–1241.23. Nofech-Mozes S, Ackerman I, Ghorab Z, Ismiil N, Thomas G, Covens A, et al. Lymphovascular invasion is a significant predictor for distant recurrence in patients with early-stage endometrial endometrioid adenocarcinoma. Am J Clin Pathol. 2008. 129:912–917.24. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer: a Gynecologic Oncology Group study. Cancer. 1987. 60:2035–2041.25. Mariani A, Webb MJ, Keeney GL, Lesnick TG, Podratz KC. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002. 87:274–280.26. Greven KM, Randall M, Fanning J, Bahktar M, Duray P, Peters A, et al. Patterns of failure in patients with stage I, grade 3 carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 1990. 19:529–534.27. Long KC, Zhou Q, Hensley ML, Alektiar KM, Gomez J, Gardner GJ, et al. Patterns of recurrence in 1988 FIGO stage IC endometrioid endometrial cancer. Gynecol Oncol. 2012. 125:99–102.28. Lin LL, Grigsby PW, Powell MA, Mutch DG. Definitive radiotherapy in the management of isolated vaginal recurrences of endometrial cancer. Int J Radiat Oncol Biol Phys. 2005. 63:500–504.29. Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomized trial. Lancet. 2010. 375:816–823.30. Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009. 27:3547–3556.31. Horowitz NS, Peters WA 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002. 99:235–240.32. Ng TY, Nicklin JL, Perrin LC, Cheuk R, Crandon AJ. Postoperative vaginal vault brachytherapy for node-negative stage II (occult) endometrial carcinoma. Gynecol Oncol. 2001. 81:193–195.33. Chadha M, Nanavati PJ, Liu P, Fanning J, Jacobs A. Patterns of failure in endometrial carcinoma stage IB grade 3 and IC patients treated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 1999. 75:103–107.34. McCloskey SA, Tchabo NE, Malhotra HK, Odunsi K, Rodabaugh K, Singhal P, et al. Adjuvant vaginal brachytherapy alone for high risk localized endometrial cancer as defined by the three major randomized trials of adjuvant pelvic radiation. Gynecol Oncol. 2010. 116:404–407.35. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009. 373:125–136.36. Convery PA, Cantrell LA, Di Santo N, Broadwater G, Modesitt SC, Secord AA, et al. Retrospective review of an intraoperative algorithm to predict lymph node metastasis in low-grade endometrial adenocarcinoma. Gynecol Oncol. 2011. 123:65–70.37. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000. 182:1506–1519.38. Fotiou S, Trimble EL, Papakonstantinou K, Kondi-Pafiti A, Panoskaltsis T, Deliconstantinos G, et al. Complete pelvic lymphadenectomy in patients with clinical early, grade I and II endometrioid corpus cancer. Anticancer Res. 2009. 29:2781–2785.39. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008. 108:226–233.40. Landrum LM, Mannel RS, Moore KN, Walker JL, Syzek EJ, Zuna RE, et al. Vaginal cuff brachytherapy combined with carboplatin and paclitaxel as adjuvant therapy for high-intermediate-risk patients with endometrial carcinoma. J Clin Oncol. 2010. 28:15s. (abstr 5095).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of simultaneous presentation of uterine endometrial adenocarcinoma with right ovarian endometrioid carcinoma and left ovarian serous adenocarcinoma
- Differential Diagnosis of Ovarian Mucinous, Serous, and Endometrioid Adenocarcinoma in Peritoneal Washing Cytology
- Role of systematic lymphadenectomy in patients with intermediate to high-risk early stage endometrial cancer
- Tumor Size is Associated with Long-term Outcomes after Resection of Gastric Gastrointestinal Stromal Tumors
- Effect of Adjuvant Chemotherapy after Complete Resection for Pathologic Stage IB Lung Adenocarcinoma in High-Risk Patients as Defined by a New Recurrence Risk Scoring Model