Recent Advances in Image-enhanced Endoscopy
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Gastrointestinal Cancer Center, Soonchunhyang University Hospital, Seoul, Korea.
- 2Division of Gastroenterology, Department of Internal Medicine, College of Medicine, Kyung Hee University, Seoul, Korea. jyjang@khu.ac.kr
- 3Division of Gastroenterology, Department of Internal Medicine, Inha University Hospital, Incheon, Korea.
- KMID: 1805252
- DOI: http://doi.org/10.5946/ce.2011.44.2.65
Abstract
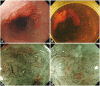
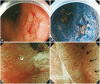
- The desire to better recognized such malignancies, which may be difficult to distinguish from inflammation or trauma, has accelerated the development of endoscopy with new optical technologies. Narrow-band imaging is a novel endoscopic technique that may enhance the accuracy of diagnosis using narrow-bandwidth filters in a red-green-blue sequential illumination system. Autofluorescence imaging is based on the detection of natural tissue fluorescence emitted by endogenous molecules. I-scan technology using a digital filter that modifies normal images through software functions, is the newly developed image-enhanced endoscopic technology from PENTAX. Flexible spectral imaging color enhancement enhances the visualization of mucosal structure and microcirculation by the selection of spectral transmittance with a dedicated wavelength. Confocal laser endomicroscopy images were collected with an argon beam with a scanning depth of 0 (epithelium) to 250 microm (lamina propria) and analyzed using the reflected light.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Increased Detection of Colorectal Polyps in Screening Colonoscopy Using High Definition i-SCAN Compared with Standard White Light
Woo Jung Kim, Sang Young Park, Iksoo Park, Wook Jin Lee, Jaechan Park, Nuri Chon, Tak Geun Oh, Kwang Hyun Kim
Clin Endosc. 2016;49(1):69-75. doi: 10.5946/ce.2016.49.1.69.Molecular Imaging for Theranostics in Gastroenterology: One Stone to Kill Two Birds
Kwang Hyun Ko, Chang-Il Kown, Jong Min Park, Hoo Geun Lee, Na Young Han, Ki Baik Hahm
Clin Endosc. 2014;47(5):383-388. doi: 10.5946/ce.2014.47.5.383.Image Quality Analysis of Various Gastrointestinal Endoscopes: Why Image Quality Is a Prerequisite for Proper Diagnostic and Therapeutic Endoscopy
Weon Jin Ko, Pyeong An, Kwang Hyun Ko, Ki Baik Hahm, Sung Pyo Hong, Joo Young Cho
Clin Endosc. 2015;48(5):374-379. doi: 10.5946/ce.2015.48.5.374.The Past, Present, and Future of Image-Enhanced Endoscopy
Jae-Young Jang
Clin Endosc. 2015;48(6):466-475. doi: 10.5946/ce.2015.48.6.466.
Reference
-
1. Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Dig Endosc. 2002; 14:131–137.
Article2. Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett's esophagus: a prospective randomized crossover study. Endoscopy. 2005; 37:929–936. PMID: 16189764.
Article3. Yasushi S, Manabu M, Hisao T, Atsushi O, Shigeaki Y. Optical/digital chromoendoscopy during colonoscopy using narrow-band imaging system. Dig Endosc. 2005; 17(Suppl 1):S43–S48.4. Muto M, Katada C, Sano Y, Yoshida S. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol. 2005; 3(7 Suppl 1):S16–S20. PMID: 16012987.
Article5. Singh S, Sharma P. Magnification endoscopy in the upper GI tract. Dig Endosc. 2005; 17(Suppl 1):S17–S19.
Article6. Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut. 2003; 52:24–27. PMID: 12477754.
Article7. Hamamoto Y, Endo T, Nosho K, Arimura Y, Sato M, Imai K. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett's esophagus. J Gastroenterol. 2004; 39:14–20. PMID: 14767729.
Article8. Inoue H, Honda T, Yoshida T, et al. Ultra-high magnification endoscopy of the normal esophageal mucosa. Dig Endosc. 1996; 8:134–138.
Article9. Inoue H, Honda T, Nagai K, et al. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1997; 9:16–18.
Article10. Inoue H. Magnification endoscopy in the esophagus and stomach. Dig Endosc. 2001; 13(Suppl 1):S40–S41.
Article11. Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004; 59:288–295. PMID: 14745410.
Article12. Capelle LG, Haringsma J, de Vries AC, et al. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010; 55:3442–3448. PMID: 20393882.
Article13. Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007; 39:202–207. PMID: 17273960.
Article14. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006; 38:819–824. PMID: 17001572.
Article15. Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008; 68:574–580. PMID: 18656862.
Article16. Yao K, Yao T, Iwashita A. Determining the horizontal extent of early gastric carcinoma: two modern techniques based on differences in the mucosal microvascular architecture and density between carcinomatous and non-carcinomatous mucosa. Dig Endosc. 2002; 14(Suppl 1):S83–S87.
Article17. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084. PMID: 15578298.
Article18. Yagi K, Nakamura A, Sekine A, Hajime U. Magnifying endoscopy with narrow band imaging for early differentiated gastric adenocarcinoma. Dig Endosc. 2008; 20:115–122.
Article19. Kadowaki S, Tanaka K, Toyoda H, et al. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009; 24:1625–1630. PMID: 19788603.
Article20. Kiyotoki S, Nishikawa J, Satake M, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010; 25:1636–1641. PMID: 20880172.
Article21. Ezoe Y, Muto M, Horimatsu T, et al. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010; 71:477–484. PMID: 20189506.
Article22. Kato M, Kaise M, Yonezawa J, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010; 72:523–529. PMID: 20598685.
Article23. Cho JY, Hong SJ. Autofluorescence imaging: as a new method for predicting metachronous gastric cancer. J Gastroenterol Hepatol. 2010; 25:1814–1815. PMID: 21091990.
Article24. Otani A, Amano Y, Koshino K, et al. Is autofluorescence imaging endoscopy useful for determining the depth of invasion in gastric cancer? Digestion. 2010; 81:96–103. PMID: 20068309.
Article25. Nakamura M, Tahara T, Shibata T, et al. Diagnostic efficacy of autofluorescence and reflectance imaging endoscopy for lateral extension of early gastric cancers. Gastrointest Endosc. 2009; 70:599. PMID: 19699985.
Article26. Kim WJ, Cho JY, Jeong SW, et al. Comparison of autofluorescence imaging endoscopic findings with pathologic findings after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2008; 2:186–192. PMID: 20485645.
Article27. Kato M, Uedo N, Ishihara R, et al. Analysis of the color patterns of early gastric cancer using an autofluorescence imaging video endoscopy system. Gastric Cancer. 2009; 12:219–224. PMID: 20047127.
Article28. Kara MA, Peters FP, Fockens P, ten Kate FJ, Bergman JJ. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett's esophagus. Gastrointest Endosc. 2006; 64:176–185. PMID: 16860064.
Article29. Ohkawa A, Miwa H, Namihisa A, et al. Diagnostic performance of light-induced fluorescence endoscopy for gastric neoplasms. Endoscopy. 2004; 36:515–521. PMID: 15202048.30. Ignjatovic A, East JE, Guenther T, et al. What is the most reliable imaging modality for small colonic polyp characterization? Study of white-light, autofluorescence, and narrow-band imaging. Endoscopy. 2011; 43:94–99. PMID: 21271465.
Article31. Lee BI. EPK-i endoscopy. Korean J Gastrointest Endosc. 2009; 39(Suppl 1):184–186.32. Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010; 16:1043–1049. PMID: 20205272.
Article33. Hoffman A, Kagel C, Goetz M, et al. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-scan is as precise as chromoendoscopy. Dig Liver Dis. 2010; 42:45–50. PMID: 19473893.
Article34. Hoffman A, Sar F, Goetz M, et al. High definition plus colonoscopy combined with i-scan is superior in the detection and characterization of colorectal neoplasias compared to standard video colonoscopy: a prospective randomized trial. Gastrointest Endosc. 2009; 69:AB131–AB132.35. Hong SW, Cho WY, Cho JY, et al. Comparison between i scan and ch-romoscopy for delineation of the margin in early gastric cancer. Endoscopy. 2011; Forthcoming.36. Yoshizawa M, Osawa H, Yamamoto H, et al. Diagnosis of elevated-type early gastric cancers by the optimal band imaging system. Gastrointest Endosc. 2009; 69:19–28. PMID: 19111685.
Article37. Osawa H, Yoshizawa M, Yamamoto H, et al. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc. 2008; 67:226–234. PMID: 18061596.
Article38. Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004; 127:706–713. PMID: 15362025.
Article39. Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006; 4:979–987. PMID: 16843068.
Article40. Liu H, Li YQ, Yu T, et al. Confocal endomicroscopy for in vivo detection of microvascular architecture in normal and malignant lesions of upper gastrointestinal tract. J Gastroenterol Hepatol. 2008; 23:56–61. PMID: 18028347.
Article41. Liu H, Li YQ, Yu T, et al. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009; 41:99–106. PMID: 19214886.
Article42. Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011; 74:772–780. PMID: 21802680.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Image-Enhanced Endoscopy Useful for the Diagnosis and Treatment of Gastrointestinal Tumor?
- Role of Image-Enhanced Endoscopy in Pancreatobiliary Diseases
- Preparation of image databases for artificial intelligence algorithm development in gastrointestinal endoscopy
- Current status of image-enhanced endoscopy in inflammatory bowel disease
- The Past, Present, and Future of Image-Enhanced Endoscopy