Cancer Res Treat.
2011 Mar;43(1):32-41.
Clinical Responses and Prognostic Indicators of Concurrent Chemoradiation for Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Radiation Oncology, The Catholic University of Korea School of Medicine, Seoul, Korea. yeonkim7@catholic.ac.kr
- 2Department of Medical Oncology, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 3Department of Medical Pulmonology, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 4Department of Radiology, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 5Department of Nuclear Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 6Department of Thoracic Surgery, The Catholic University of Korea School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To evaluate treatment outcomes and prognostic factors in non-small cell lung cancer (NSCLC) patients treated with concurrent chemoradiation.
MATERIALS AND METHODS
From January 2005 to June 2009, 51 patients were treated with concurrent chemoradiation for 3 different aims: locally advanced stage III, locally recurrent disease, and postoperative gross residual NSCLC. Median age was 63 years. Distribution of stages by the 6th edition of American Joint Committee on Cancer (AJCC) was as follows: IIIA (37.3%), IIIB (56.9%). Chemotherapy was administered every week concurrently with radiation using one of the following regimens: paclitaxel (60 mg/m2), docetaxel+cisplatin (20 mg/m2+20 mg/m2), cisplatin (30 mg/m2). Total radiation dose was 16-66.4 Gy (median, 59.4 Gy).
RESULTS
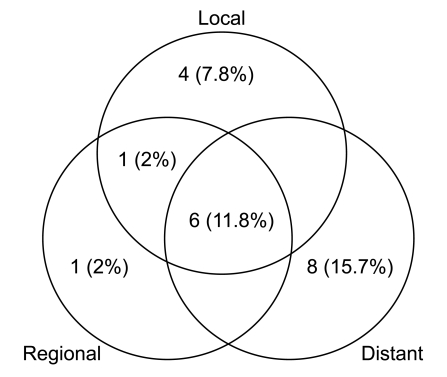
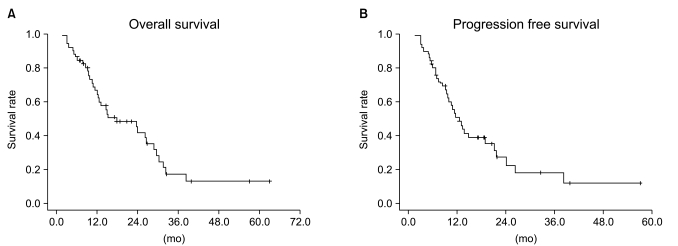
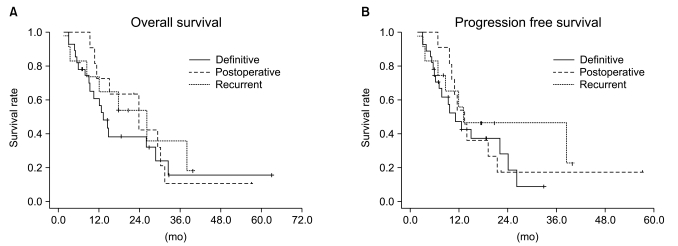
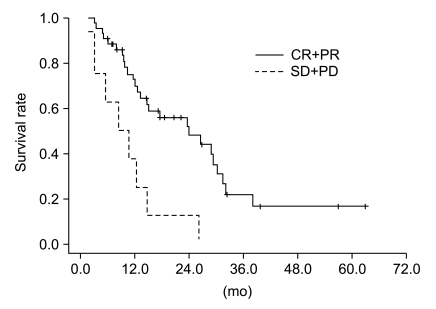
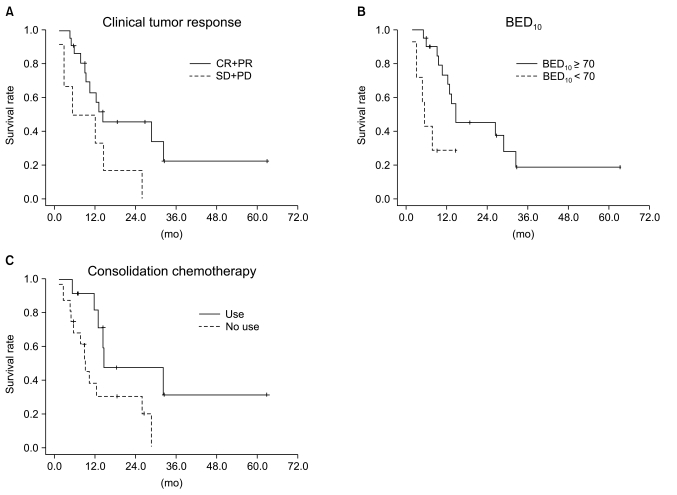
Median follow-up duration was 40.8 months. The overall response rate was 84.3% with 23 complete responses. The median survival duration for the overall patient group was 17.6 months. The 3-year survival rate was 17.8%. A total of 21 patients had recurrent disease at the following sites: loco-regional sites (23.6%), distant organs (27.5%). In the multivariate analysis of the overall patient group, a clinical tumor response (p=0.002) was the only significant prognostic factor for overall survival (OS). In the multivariate analysis of the definitive chemoradiation arm, the use of consolidation chemotherapy (p=0.022), biologically equivalent dose (BED)10 (p=0.007), and a clinical tumor response (p=0.030) were the significant prognostic factors for OS.The median survival duration of the locally recurrent group and the postoperative gross residual group were 26.4 and 23.9 months, respectively.
CONCLUSION
Our study demonstrated that clinical tumor response was significantly associated with OS in the overall patient group. Further investigations regarding the optimal radiation dose in the definitive chemoradiation and the optimal treatment scheme in locally recurrent NSCLC would be required.
Keyword
MeSH Terms
Figure
Reference
-
1. Korea Central Cancer Registry. Annual report of causes of deaths, 2009. 2010. Seoul: Ministry of Health and Welfare.2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.
Article3. Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990; 323:940–945. PMID: 2169587.
Article4. Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991; 83:417–423. PMID: 1847977.
Article5. Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996; 88:1210–1215. PMID: 8780630.
Article6. Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol. 2007; 25:313–318. PMID: 17235046.
Article7. Albain KS, Crowley JJ, Turrisi AT 3rd, Gandara DR, Farrar WB, Clark JI, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002; 20:3454–3460. PMID: 12177106.
Article8. Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004; 46:87–98. PMID: 15364136.
Article9. Socinski MA, Morris DE, Halle JS, Moore DT, Hensing TA, Limentani SA, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004; 22:4341–4350. PMID: 15514375.
Article10. Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN Jr, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003; 21:2004–2010. PMID: 12743155.
Article11. Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008; 26:5755–5760. PMID: 19001323.
Article12. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009; 374:379–386. PMID: 19632716.
Article13. Leung J, Ball D, Worotniuk T, Laidlaw C. Survival following radiotherapy for post-surgical locoregional recurrence of non-small cell lung cancer. Lung Cancer. 1995; 13:121–127. PMID: 8581391.
Article14. Kelsey CR, Clough RW, Marks LB. Local recurrence following initial resection of NSCLC: salvage is possible with radiation therapy. Cancer J. 2006; 12:283–288. PMID: 16925972.15. Foo K, Gebski V, Yeghiaian-Alvandi R, Foroudi F, Cakir B. Outcome following radiotherapy for loco-regionally recurrent non-small cell lung cancer. Australas Radiol. 2005; 49:108–112. PMID: 15845045.16. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999; 17:2692–2699. PMID: 10561343.
Article17. Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005; 23:5910–5917. PMID: 16087956.
Article18. Ahn SJ, Kim YC, Kim KS, Park KO, Chung WK, Nam TK, et al. Results of curative radiation therapy with or without chemotherapy for stage III unresectable non-small cell lung cancer. Cancer Res Treat. 2005; 37:268–272. PMID: 19956525.
Article19. Mudad R, Ramsey M, Kovitz K, Curiel TJ, Hartz R, Nedzi LL, et al. Concomitant weekly docetaxel, cisplatin and radiation therapy in locally advanced non-small cell lung cancer: a dose finding study. Lung Cancer. 2003; 39:173–177. PMID: 12581570.
Article20. Firat S, Byhardt RW, Gore E. Radiation Therapy Oncology Group. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Int J Radiat Oncol Biol Phys. 2002; 54:357–364. PMID: 12243808.
Article21. Ademuyiwa FO, Johnson CS, White AS, Breen TE, Harvey J, Neubauer M, et al. Prognostic factors in stage III non-small-cell lung cancer. Clin Lung Cancer. 2007; 8:478–482. PMID: 17922971.
Article22. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008; 26:2450–2456. PMID: 18378568.
Article23. Park K, Ahn Y, Chen M, Cho E, Kim J, Min Y, et al. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN): interim analysis. J Clin Oncol. 2009; 27(15s):7538.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Chemoradiation with Weekly Paclitaxel in Locally Advanced Non-small Cell Lung Cancer
- Thoracic Radiotherapy Combined with Chemotherapy in Patients with Limited-stage Small-cell Lung Cancer
- Current Update on the Management of Locally Advanced Non-small Cell Lung Cancer
- Clinical study on therapeutic responses and prognostic significance according to histologic subtypes of small cell lung cancer
- Unraveling the Impact of Sarcopenia-Induced Lymphopenia on Treatment Response and Prognosis in Patients with Stage III Non–Small Cell Lung Cancer: Insights for Optimizing Chemoradiation and Immune Checkpoint Inhibitor