Cancer Res Treat.
2008 Sep;40(3):133-140.
Clinical Significance of Vascular Endothelial Growth Factors (VEGF)-C and -D in Resected Non-Small Cell Lung Cancer
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. y331@ catholic.ac.kr
- 2Department of Hospital Pathology, Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 3Division of Oncology, Department of Internal Medicine, St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 4Department of Chest Surgery, Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
Abstract
-
PURPOSE: Lymphatic spread of tumor is an important prognostic factor for patients with non-small cell lung carcinoma (NSCLC). Vascular endothelial growth factor-C (VEGF-C) and VEGF-D play important roles in lymphangiogenesis via the VEGF receptor 3 (VEGFR-3). We sought to determine whether VEGF-C, VEGF-D and VEGFR-3 are involved in the clinical outcomes of patients with resected NSCLC.
MATERIALS AND METHODS
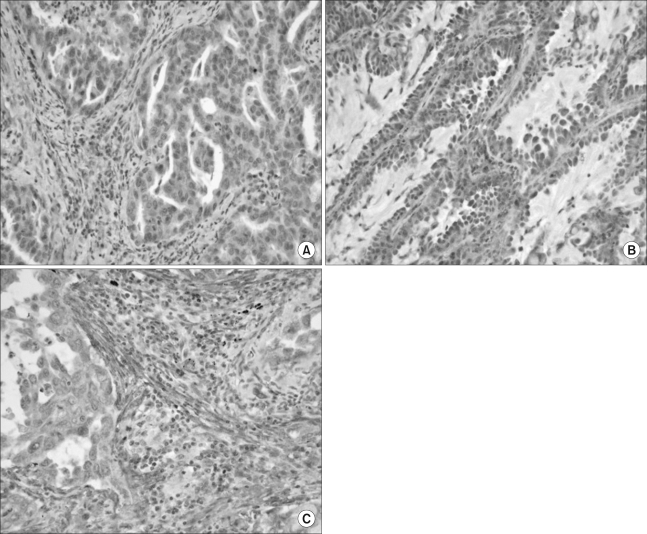
Using immunohistochemical staining, we investigated the protein expressions of VEGF-C, VEGF-D and VEGFR-3 in the tissue array specimens from patients who underwent resection for NSCLC. The immunoreactivity for p53 was also examined. The clinicopathological implications of these molecules were statistically analyzed.
RESULTS
Analysis of a total of 118 specimens showed that VEGF-C, VEGF-D and their co-expression were significantly associated with more advanced regional lymph node metastasis (p=0.019, p=0.044 and p=0.026, respectively, N2 versus N0 and N1). A VEGFR-3 expression had a strong correlation with peritumoral lymphatic invasion (p=0.047). On the multivariate analysis for survival and recurrence, pathologic N2 lymph node metastasis was the only independent prognostic factor, but none of the investigated molecules showed any statistical correlation with recurrence and survival.
CONCLUSIONS
The present study revealed that high expressions of VEGF-C and VEGF-D were strongly associated with more advanced regional lymph node metastasis in patients with resected NSCLC.
Keyword
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung
Humans
Lung
Lymph Nodes
Lymphangiogenesis
Multivariate Analysis
Neoplasm Metastasis
Receptors, Vascular Endothelial Growth Factor
Recurrence
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor C
Vascular Endothelial Growth Factor D
Vascular Endothelial Growth Factor Receptor-3
Vascular Endothelial Growth Factors
Receptors, Vascular Endothelial Growth Factor
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor C
Vascular Endothelial Growth Factor D
Vascular Endothelial Growth Factor Receptor-3
Vascular Endothelial Growth Factors
Figure
Reference
-
1. Park K. Second-line chemotherapy for advanced non-small cell lung cancer: past, present, and hope for the future. Cancer Res Treat. 2003; 35:279–280.
Article2. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997; 111:1710–1717. PMID: 9187198.
Article3. Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Yokose T, et al. Conventional clinicopathologic prognostic factors in surgically resected nonsmall cell lung carcinoma. A comparison of prognostic factors for each pathologic TNM stage based on multivariate analyses. Cancer. 1999; 86:1976–1984. PMID: 10570421.4. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002; 2:573–583. PMID: 12154350.
Article5. White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, et al. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002; 62:1669–1675. PMID: 11912138.6. Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003; 88:237–244. PMID: 12610509.
Article7. Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006; 24:228–240. PMID: 16344322.8. Okada K, Osaki M, Araki K, Ishiguro K, Ito H, Ohgi S. Expression of hypoxia-inducible factor (HIF-1alpha), VEGF-C and VEGF-D in non-invasive and invasive breast ductal carcinomas. Anticancer Res. 2005; 25:3003–3009. PMID: 16080559.9. Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999; 154:1381–1390. PMID: 10329591.
Article10. Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003; 97:457–464. PMID: 12518370.
Article11. Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000; 60:203–212. PMID: 10667560.12. Shaulsky G, Goldfinger N, Rotter V. Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res. 1991; 51:5232–5237. PMID: 1717142.13. Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004; 64:9027–9034. PMID: 15604268.
Article14. Mylona E, Alexandrou P, Mpakali A, Giannopoulou I, Liapis G, Markaki S, et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol. 2007; 33:294–300. PMID: 17129704.
Article15. Adachi Y, Nakamura H, Kitamura Y, Taniguchi Y, Araki K, Shomori K, et al. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol Int. 2007; 57:171–177. PMID: 17316411.
Article16. Kleespies A, Bruns CJ, Jauch KW. Clinical significance of VEGF-A, -C and -D expression in esophageal malignancies. Onkologie. 2005; 28:281–288. PMID: 15867486.
Article17. Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, et al. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005; 10:318–327. PMID: 16247658.
Article18. Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005; 65:110–116. PMID: 15880525.
Article19. Ishii H, Yazawa T, Sato H, Suzuki T, Ikeda M, Hayashi Y, et al. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs). Lung Cancer. 2004; 45:325–337. PMID: 15301873.
Article20. Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, et al. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol. 2007; 170:1348–1361. PMID: 17392173.
Article21. Tomita M, Matsuzaki Y, Shimizu T, Hara M, Ayabe T, Onitsuka T. Vascular endothelial growth factor expression in pN2 non-small cell lung cancer: lack of prognostic value. Respirology. 2005; 10:31–35. PMID: 15691235.
Article22. Liao M, Wang H, Lin Z, Feng J, Zhu D. Vascular endothelial growth factor and other biological predictors related to the postoperative survival rate on non-small cell lung cancer. Lung Cancer. 2001; 33:125–132. PMID: 11551407.
Article23. Tsubochi H, Sato N, Hiyama M, Kaimori M, Endo S, Sohara Y, et al. Combined analysis of cyclooxygenase-2 expression with p53 and Ki-67 in nonsmall cell lung cancer. Ann Thorac Surg. 2006; 82:1198–1204. PMID: 16996907.
Article24. Katsuta M, Miyashita M, Makino H, Nomura T, Shinji S, Yamashita K, et al. Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol. 2005; 78:123–130. PMID: 15713437.25. Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005; 201:93–99. PMID: 15901129.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Role of Vascular Endothelial Growth Factor (VEGF) Expression and Angiogenesis in Curatively Resected Non-Small Cell Lung Cancer
- Prognostic Value of Vascular Endothelial Growth Factor(VEGF) in Resected Non-Small Cell Lung Cancer
- Immunohistochemical Expression and Prognostic Value of VEGF, HIF-1alpha, EGFR in Non-Small Cell Lung Cancer
- Expression of p53 and Vascular Endothelial Growth Factor mRNA in Angiogenesis of Non-Small Cell Lung Carcinoma
- Immunohistochemical Study of C-erbB-2 and VEGF Expression in Non-Small Cell Lung Cancer