J Korean Ophthalmol Soc.
2015 Feb;56(2):174-179. 10.3341/jkos.2015.56.2.174.
The Effect of Topical Cyclosporine 0.05% on Tear Osmolarity for Dry Eye Syndrome
- Affiliations
-
- 1Nune Eye Hospital, Seoul, Korea. thchoi@hanmail.net
- KMID: 2215999
- DOI: http://doi.org/10.3341/jkos.2015.56.2.174
Abstract
- PURPOSE
To evaluate the effect of topical cyclosporine 0.05% (Restasis; Allergan, Irving, CA, USA) on tear osmolarity in patients with dry eye disease.
METHODS
The present study was a single-center, randomized, prospective, and longitudinal trial. Patients who had been using artificial tears to treat dry eye disease were prescribed cyclosporine 0.05% and evaluated using tear osmolarity, tear break-up time, ocular surface staining score, Schirmer test, and the Ocular Surface Disease Index for symptomatic improvement. Clinical measurements of commonly used objective tests were performed at baseline and after 1, 3, and 6 months.
RESULTS
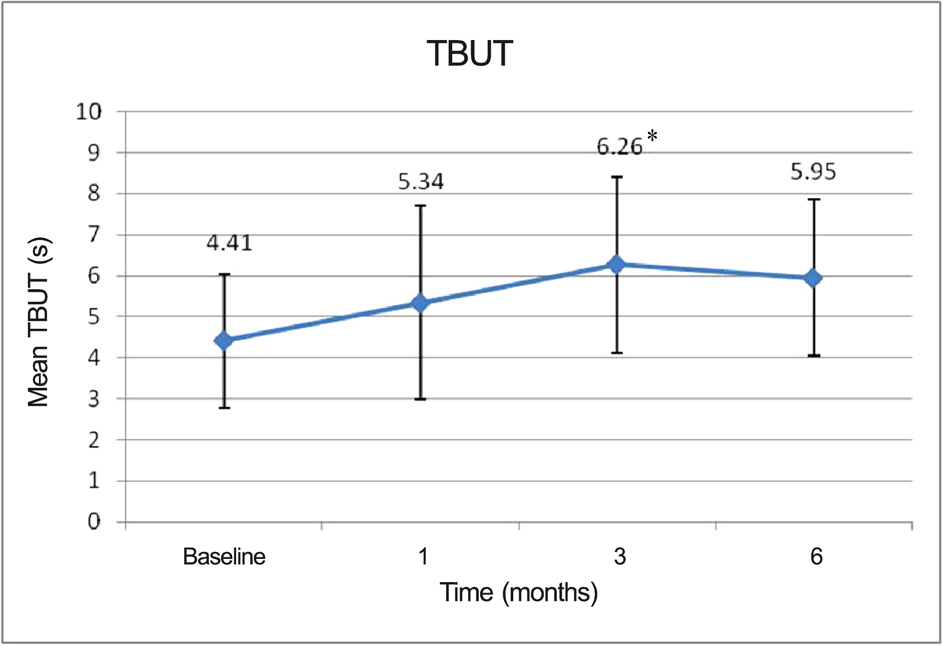
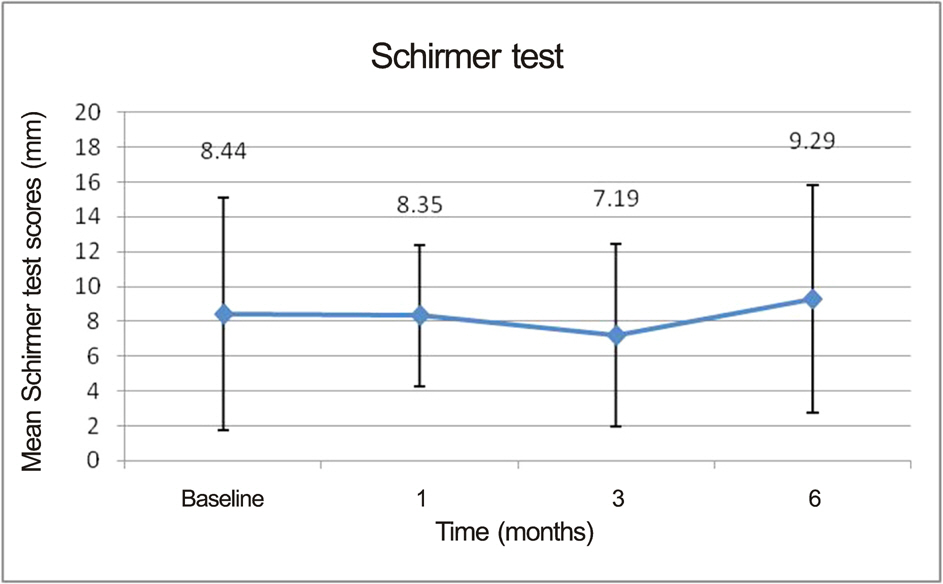
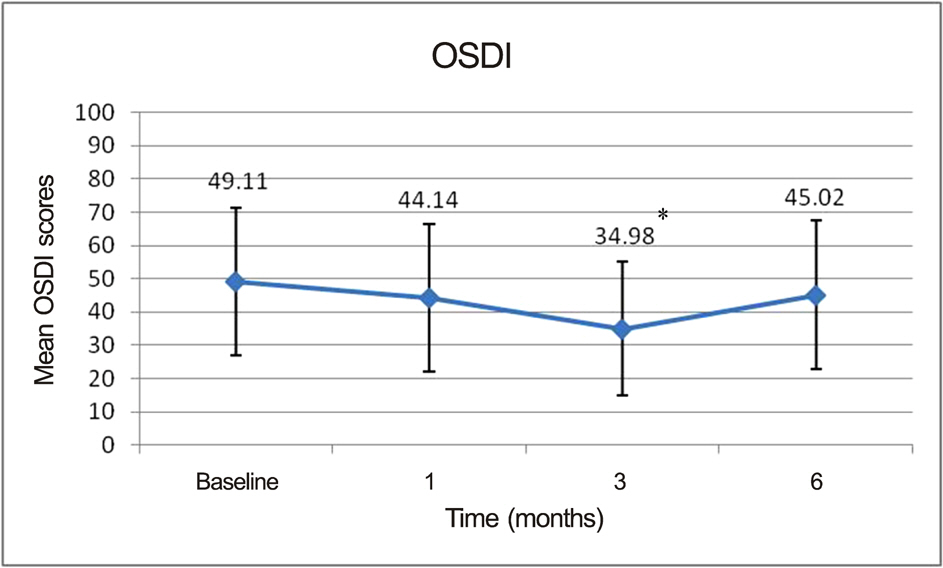
At the end of the study, patients demonstrated statistically significant improvement in tear break-up time (6.26 +/- 1.26 sec at 3 months vs. 4.41 +/- 1.63 sec at baseline, p = 0.022) and OSDI (34.98 +/- 20.19 at 3 months vs. 45.02 +/- 22.38 at baseline, p = 0.032) only at 3 months. Other measures such as Schirmer test, ocular surface grade, and tear osmolarity also showed improvement. However, the differences were not significant.
CONCLUSIONS
Over a 6-month period, topical cyclosporine 0.05% showed beneficial effects on symptoms and other commonly used signs of dry eye disease for 3 months; however, the tear osmolarity values were not significantly improved.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:179–93.2. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine oph-thalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.3. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.4. Perry HD, Solomon R, Donnenfeld ED. . Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008; 126:1046–50.
Article5. Hyon JY, Kim HM, Lee D. . Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014; 28:197–206.
Article6. Schiffman RM, Christianson MD, Jacobsen G. . Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118:615–21.
Article7. Nasu M, Matsubara O, Yamamoto H. Post-mortem prevalence of lymphocytic infiltration of the lacrymal gland: a comparative study in autoimmune and non-autoimmune diseases. J Pathol. 1984; 143:11–5.
Article8. Pepose JS, Akata RF, Pflugfelder SC, Voigt W. Mononuclear cell phenotypes and immunoglobulin gene rearrangements in lacrimal gland biopsies from patients with Sjögren's syndrome. Ophthalmology. 1990; 97:1599–605.
Article9. Pflugfelder SC, Jones D, Ji Z. . Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome ker-atoconjunctivitis sicca. Curr Eye Res. 1999; 19:201–11.
Article10. Afonso AA, Sobrin L, Monroy DC. . Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999; 40:2506–12.11. Solomon A, Dursun D, Liu Z. . Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dryeye disease. Invest Ophthalmol Vis Sci. 2001; 42:2283–92.12. McCollum CJ, Foulks GN, Bodner B. . Rapid assay of lactoferrin in keratoconjunctivitis sicca. Cornea. 1994; 13:505–8.
Article13. Furuichi S, Hashimoto S, Gon Y. . p38 mitogen-activated pro-tein kinase and c-Jun-NH2-terminal kinase regulate interleukin-8 and RANTES production in hyperosmolarity stimulated human bronchial epithelial cells. Respirology. 2002; 7:193–200.
Article14. Li DQ, Chen Z, Song XJ. . Stimulation of matrix metal-loproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45:4302–11.
Article15. Luo L, Li DQ, Doshi A. . Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004; 45:4293–301.
Article16. Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996; 274:1194–7.
Article17. Li DQ, Luo L, Chen Z. . JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyper-osmolar stress in human limbal epithelial cells. Exp Eye Res. 2006; 82:588–96.18. Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008; 27:64–9.19. Brignole F, Pisella PJ, De Saint Jean M. . Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001; 42:90–5.20. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000; 47:119–25.
Article21. Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007; 26:452–60.
Article22. Chen Z, Tong L, Li Z. . Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008; 49:539–49.
Article23. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005; 24:80–5.
Article24. Gupta A, Monroy D, Ji Z. . Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996; 15:605–14.
Article25. Yoshino K, Garg R, Monroy D. . Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr Eye Res. 1996; 15:615–24.26. Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009; 54:321–38.27. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004; 137:337–42.
Article28. Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009; 7:199–211.
Article29. Lemp MA, Bron AJ, Baudouin C. . Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011; 151:792–798.e1.
Article30. Tomlinson A, Khanal S, Ramaesh K. . Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006; 47:4309–15.
Article31. Khanal S, Tomlinson A, McFadyen A. . Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008; 49:1407–14.
Article32. Vanley GT, Leopold IH, Gregg TH. Interpretation of tear film breakup. Arch Ophthalmol. 1977; 95:445–8.
Article33. Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004; 23:272–85.
Article34. Rao SN. Reversibility of dry eye deceleration after topical cyclosporine 0.05% withdrawal. J Ocul Pharmacol Ther. 2011; 27:603–9.
Article35. Amparo F, Jin Y, Hamrah P. . What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 2014; 157:69–77.e2.
Article36. Chang IB, Park JH, Kim MS, Kim TJ. Effect of sodium hyaluro-nate and cyclosporine A on tear film in dry eye syndrome. J Korean Ophthalmol Soc. 2013; 54:231–6.
Article37. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999; 106:811–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Sodium Hyaluronate and Cyclosporine A on Tear Film in Dry Eye Syndrome
- Efficacy of Topical Cyclosporine in Mild Dry Eye Patients Having Refractive Surgery
- Clinical Significance of Tear Film Osmolarity in Patients with Mild Dry Eye Syndrome
- Effect of 0.1% Sodium Hyaluronate and 0.05% Cyclosporine on Tear Film Parameters after Cataract Surgery
- The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery