Korean J Ophthalmol.
2013 Jun;27(3):149-157. 10.3341/kjo.2013.27.3.149.
Autologous Tragal Perichondrium Transplantation: A Novel Approach for the Management of Painful Bullous Keratopathy
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University Hospital, Seoul, Korea. jck50ey@kornet.net
- KMID: 1798050
- DOI: http://doi.org/10.3341/kjo.2013.27.3.149
Abstract
- PURPOSE
To introduce autologous tragal perichondrium transplantation as a novel surgical modality for the management of intractable symptomatic bullous keratopathy.
METHODS
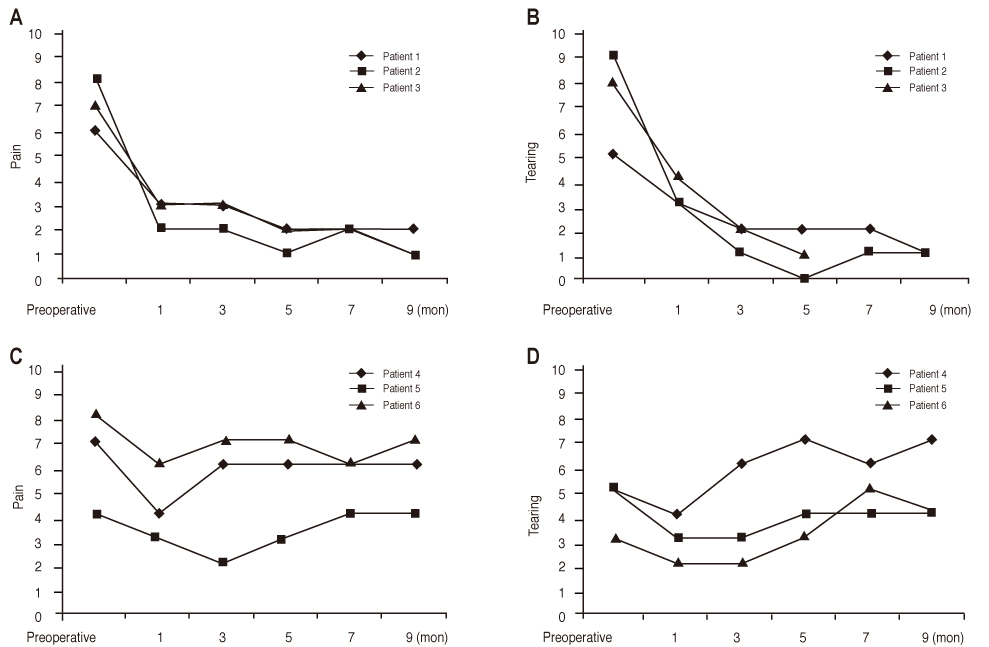
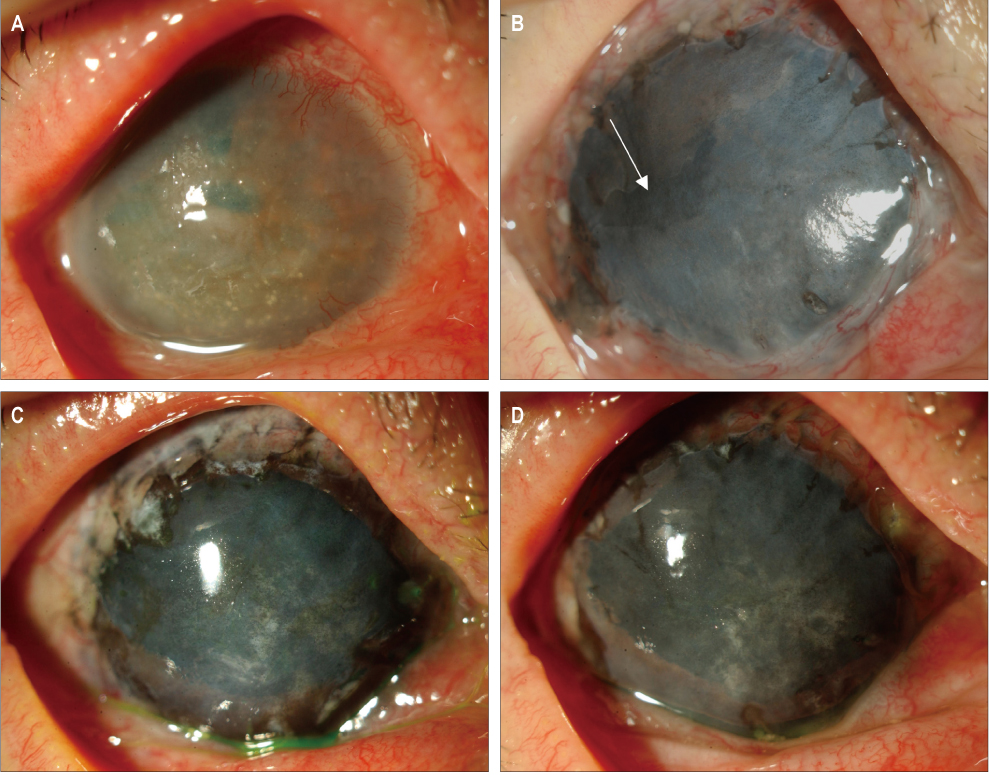
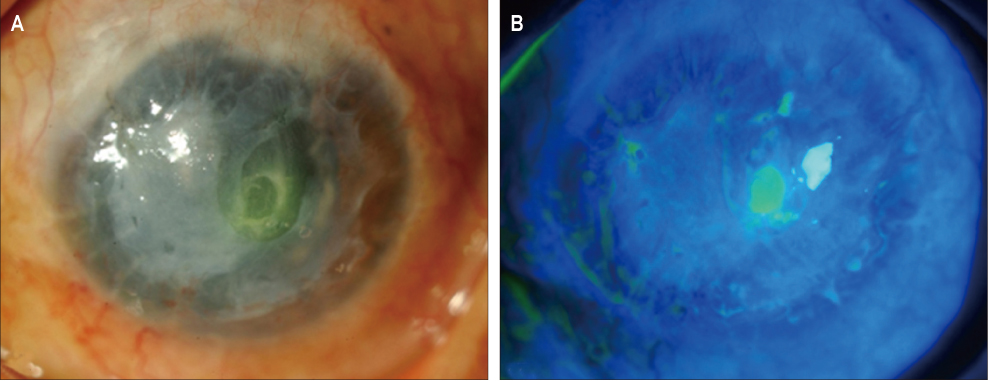
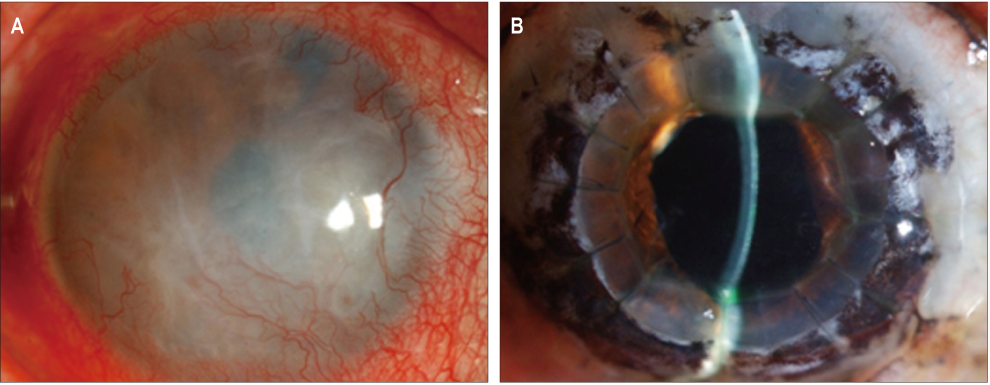
In three eyes of three patients with painful bullous keratopathy, autologous tragal perichondria were transplanted on the corneal surface with the human amniotic membrane transplanted above. We included an additional three eyes of three patients with painful bullous keratopathy who received amniotic membrane transplantation only to serve as controls. Clinical symptom outcomes were assessed using a visual analogue scale at postsurgical months 1, 3, 5, 7, and 9. In addition, transplanted tragal perichondrium and amniotic membrane complex tissue button obtained from one patient who underwent penetrating keratoplasty was evaluated by immunohistochemical analysis of CD34, vimentin, and alcian blue staining.
RESULTS
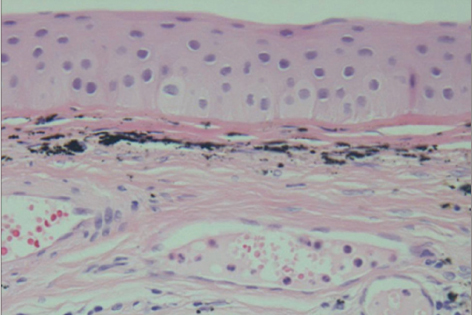
All three patients who underwent autologous tragal perichondrium and human amniotic membrane co-transplantation showed improvements in pain and tearing. However, all three patients in the control group experienced aggravation of tearing and no further improvement of pain 3 months after surgery. In addition, one patient in the control group developed premature degradation of the amniotic membrane. Histopathologic and immunohistochemical analysis showed intact surface epithelization and positive CD34, vimentin and alcian blue staining of transplanted tragal perichondria.
CONCLUSIONS
The tragal perichondrium has a high mechanical structural force and high potency due to well-organized epithelization and the presence of mesenchymal stem cells. Autologous tragal perichondrium transplantation may be an effective modality for the management of painful bullous keratopathy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Case of Autologous Tragal Perichondrium Graft in a Patient with Mooren's Ulcer
Dong Ik Lee, Kyoung Woo Kim, Jae Chan Kim
J Korean Ophthalmol Soc. 2014;55(3):437-442. doi: 10.3341/jkos.2014.55.3.437.
Reference
-
1. Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005. 81:629–638.2. Pires RT, Tseng SC, Prabhasawat P, et al. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999. 117:1291–1297.3. Gomes JA, Haraguchi DK, Zambrano DU, et al. Anterior stromal puncture in the treatment of bullous keratopathy: six-month follow-up. Cornea. 2001. 20:570–572.4. Brady SE, Rapuano CJ, Arentsen JJ, et al. Clinical indications for and procedures associated with penetrating keratoplasty, 1983-1988. Am J Ophthalmol. 1989. 108:118–122.5. Cursiefen C, Kuchle M, Naumann GO. Changing indications for penetrating keratoplasty: histopathology of 1,250 corneal buttons. Cornea. 1998. 17:468–470.6. Mamalis N, Anderson CW, Kreisler KR, et al. Changing trends in the indications for penetrating keratoplasty. Arch Ophthalmol. 1992. 110:1409–1411.7. Alino AM, Perry HD, Kanellopoulos AJ, et al. Conjunctival flaps. Ophthalmology. 1998. 105:1120–1123.8. Lamberts DW. Topical hyperosmotic agents and secretory stimulants. Int Ophthalmol Clin. 1980. 20:163–169.9. Mejía LF, Santamaria JP, Acosta C. Symptomatic management of postoperative bullous keratopathy with nonpreserved human amniotic membrane. Cornea. 2002. 21:342–345.10. Sridhar MS, Vemuganti GK, Bansal AK, Rao GN. Anterior stromal puncture in bullous keratopathy: a clinicopathologic study. Cornea. 2001. 20:573–579.11. Thomann U, Meier-Gibbons F, Schipper I. Phototherapeutic keratectomy for bullous keratopathy. Br J Ophthalmol. 1995. 79:335–338.12. Kent HD, Cohen EJ, Laibson PR, Arentsen JJ. Microbial keratitis and corneal ulceration associated with therapeutic soft contact lenses. CLAO J. 1990. 16:49–52.13. Espana EM, Grueterich M, Sandoval H, et al. Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. J Cataract Refract Surg. 2003. 29:279–284.14. Cavaliere M, Mottola G, Rondinelli M, Iemma M. Tragal cartilage in tympanoplasty: anatomic and functional results in 306 cases. Acta Otorhinolaryngol Ital. 2009. 29:27–32.15. Nigro MV, Friedhofer H, Natalino RJ, Ferreira MC. Comparative analysis of the inf luence of perichondrium on conjunctival epithelialization on conchal cartilage grafts in eyelid reconstruction: experimental study in rabbits. Plast Reconstr Surg. 2009. 123:55–63.16. Yotsuyanagi T, Urushidate S, Watanabe M, Sawada Y. Reconstruction of a three-dimensional structure using cartilage regenerated from the perichondrium of rabbits. Plast Reconstr Surg. 1999. 103:1120–1123.17. Arai F, Ohneda O, Miyamoto T, et al. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002. 195:1549–1563.18. Togo T, Utani A, Naitoh M, et al. Identification of cartilage progenitor cells in the adult ear perichondrium: utilization for cartilage reconstruction. Lab Invest. 2006. 86:445–457.19. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004. 49:51–77.20. Fernandes M, Moreker MR, Shah SG, Vemuganti GK. Exaggerated subepithelial fibrosis after anterior stromal puncture presenting as a membrane. Cornea. 2011. 30:660–663.21. Maini R, Sullivan L, Snibson GR, et al. A comparison of different depth ablations in the treatment of painful bullous keratopathy with phototherapeutic keratectomy. Br J Ophthalmol. 2001. 85:912–915.22. Madsen K, von der Mark K, van Menxel M, Friberg U. Analysis of collagen types synthesized by rabbit ear cartilage chondrocytes in vivo and in vitro. Biochem J. 1984. 221:189–196.23. Koo H, Jeong JH, Chun YS, Kim JC. Ocular reconstruction using autologous tragal perichondrium for a refractory necrotizing scleral perforation: a case report. J Korean Ophthalmol Soc. 2011. 52:1227–1231.24. Migirov L, Shapira Y, Horowitz Z, Wolf M. Exclusive endoscopic ear surgery for acquired cholesteatoma: preliminary results. Otol Neurotol. 2011. 32:433–436.25. Ahn BH, Lee EJ, Sung KH. The use of a temporal muscle fascia in the treatment of scleral defect. J Korean Ophthalmol Soc. 1983. 24:785–791.26. Kwak JY, Chang HK. Autogenous temporalis fascia grafting and conjunctival flap transposition in scleromalacia after pterygium excision. J Korean Ophthalmol Soc. 2004. 45:180–186.27. Kim JH. Scleral grafting on necrotic scleritis following pterygium excision. J Korean Ophthalmol Soc. 1982. 23:29–36.28. Lee CO, Jong SH, Lee JJ. Autologous simple conjunctival graft and conjunctiva/tenon graft on focal scleromalacia. J Korean Ophthalmol Soc. 1997. 38:1737–1741.29. Na YS, Joo MJ, Kim JH. Results of scleral allografting on scleral necrosis following pterygium excision. J Korean Ophthalmol Soc. 2005. 46:402–409.30. Mauriello JA Jr, Fiore PM, Pokorny KS, Cinotti DJ. Use of split-thickness dermal graft in the surgical treatment of corneal and scleral defects. Am J Ophthalmol. 1988. 105:244–247.31. Smith MF, Doyle JW, Ticrney JW Jr. A comparison of glaucoma drainage implant tube coverage. J Glaucoma. 2002. 11:143–147.32. Pinto LM, Regatieri CV, Tavares IM, Rigueiro MP. Porcine pericardium as glaucoma implant tube coverage: an experimental study. Arq Bras Oftalmol. 2008. 71:623–628.33. Ljung A, Ohlsen L, Widenfalk B, Gerdin B. Characterisation of cells in regenerating cartilage from autotransplanted perichondrium: immunohistochemical expression of smooth-muscle actin, desmin, vimentin, and Ki-67. Scand J Plast Reconstr Surg Hand Surg. 1999. 33:257–266.34. Borboli S, Colby K. Mechanisms of disease: Fuchs' endothelial dystrophy. Ophthalmol Clin North Am. 2002. 15:17–25.35. Tosi GM, Traversi C, Schuerfeld K, et al. Amniotic membrane graft: histopathological findings in five cases. J Cell Physiol. 2005. 202:852–857.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ocular Reconstruction Using Autologous Tragal Perichondrium for a Refractory Necrotizing Scleral Perforation: A Case Report
- Efficacy of Autologous Tragal Perichondrium Graft after Proper Antifungal Treatment in Fungal Necrotizing Scleritis
- A Case of Autologous Tragal Perichondrium Graft in a Patient with Mooren's Ulcer
- The Clinical Effects of Dye-Amniotic Membrane Transplantation on Bullous Keratopathy
- Simultaneous Four-site Restoration of Bilateral Scleromalacia Using Autologous Tragal Perichondrium and Preserved Sclera