Clin Orthop Surg.
2014 Dec;6(4):455-461. 10.4055/cios.2014.6.4.455.
The Effect of Poloxamer 407-Based Hydrogel on the Osteoinductivity of Demineralized Bone Matrix
- Affiliations
-
- 1Department of Orthopedic Surgery, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. spinelee@snu.ac.kr
- 2Institute of Medical and Biological Engineering, Medical Research Center, Seoul National University, Seoul, Korea.
- 3Department of Orthopedic Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1794735
- DOI: http://doi.org/10.4055/cios.2014.6.4.455
Abstract
- BACKGROUND
Demineralized bone matrix (DBM) is used for bone healing due to its osteoinductivity, but it requires a carrier for clinical application. Here, we report the effects on the osteoinductivity of DBM by use of a poloxamer 407-based hydrogel as the carrier, compared to sterile water.
METHODS
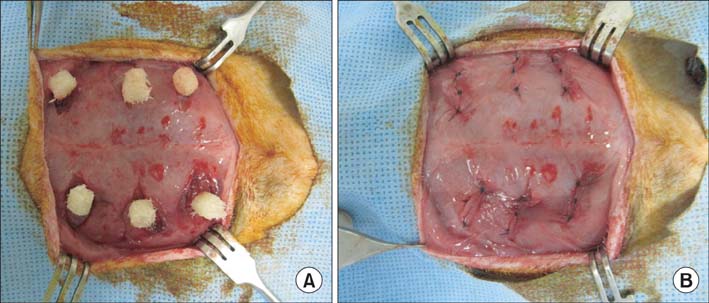
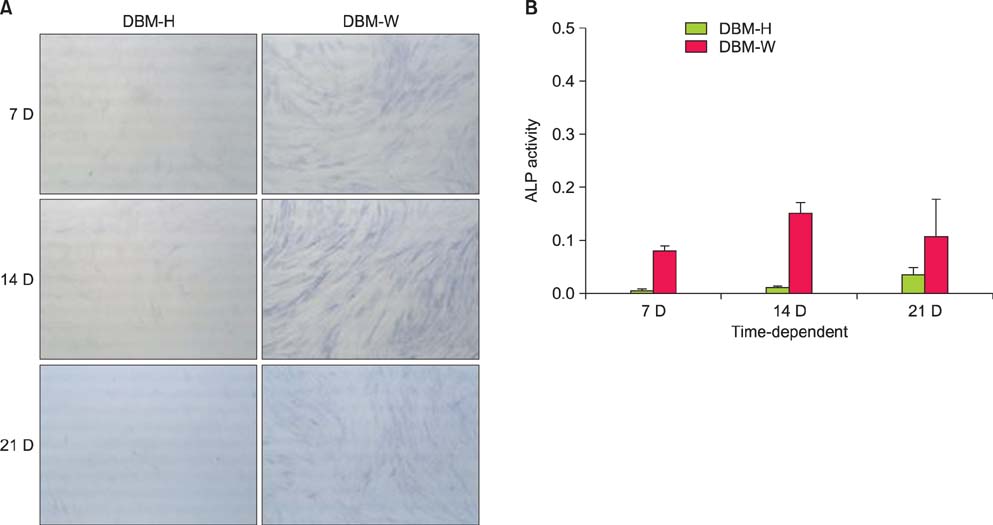
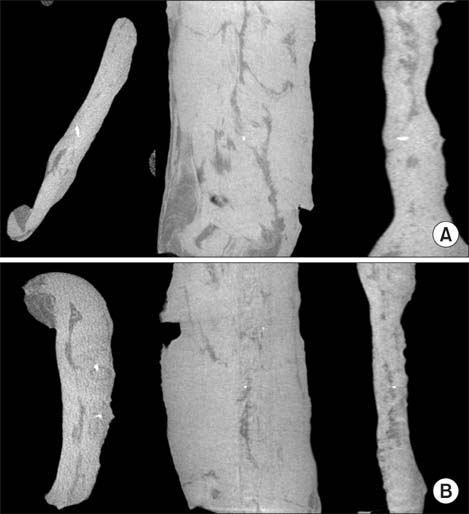
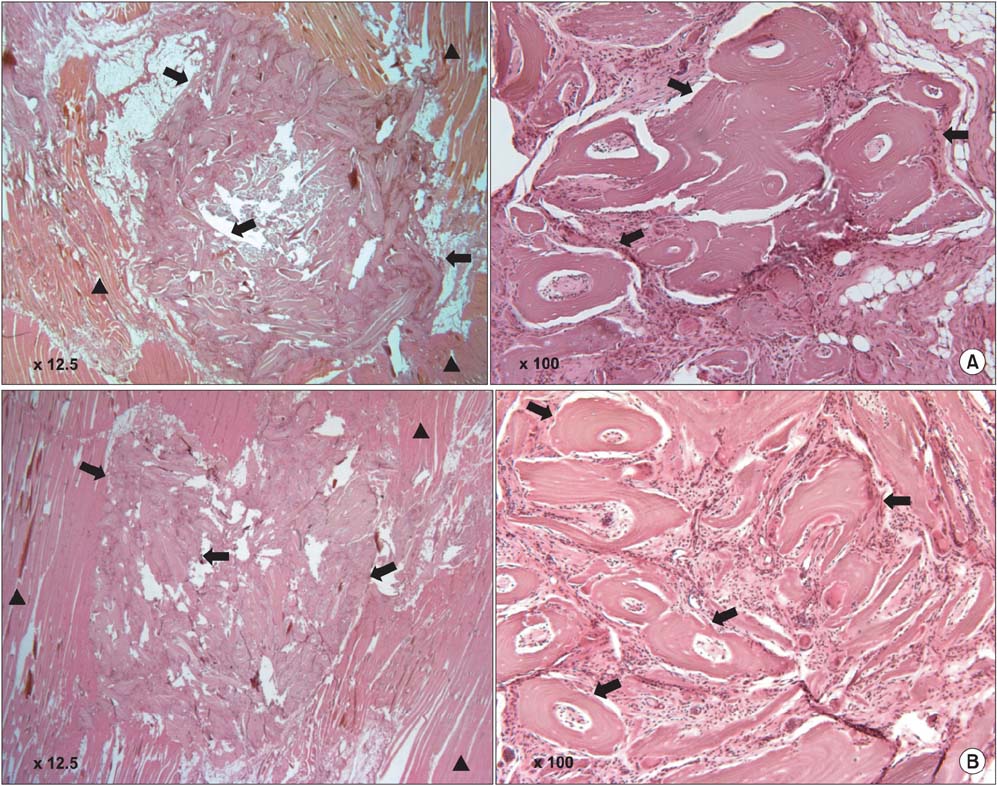
DBM-W and DBM-H represent 27 wt% of DBM with sterile water and DBM with a poloxamer 407-based hydrogel, respectively. Both of the compositions were applied to human mesenchymal stem cell (MSC) cultures, and monitored for alkaline phosphatase (ALP) staining and ALP activity. Six 10-week-old athymic nude rats were used for abdominal muscle grafting with either DBM-W or DBM-H, and were tested by plane radiography, microfocus X-ray computed tomography (CT), and decalcified histology to evaluate ectopic bone formation.
RESULTS
The DBM-W group showed stronger ALP staining at 7, 14, and 21 days of treatment, and significantly higher ALP activity at 7 and 14 days of treatment, compared to the DBM-H group. Plane radiography could not confirm the radio-opaque lesions in the rat ectopic bone formulation model. However, ectopic bone formation was observed in both groups by micro-CT. Compared to the DBM-H group, the DBM-W group showed higher bone volume, percent bone volume and trabecular number, and the difference in percent bone volume was statistically significant. Decalcified histology found bony tissue with lamellation in both groups.
CONCLUSIONS
Our results suggest that poloxamer 407-based hydrogel has efficacy as a DBM carrier since it shows ectopic bone formation, but its effects on the quality and quantity of osteoblastic differentiation in rat abdominal ectopic bone and MSC are considered negative.
MeSH Terms
Figure
Reference
-
1. Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989; 71(4):677–680.2. Lee JH, Hwang CJ, Song BW, Koo KH, Chang BS, Lee CK. prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009; 90(3):804–810.3. Gunzburg R, Szpalski M. Use of a novel beta-tricalcium phosphate-based bone void filler as a graft extender in spinal fusion surgeries. Orthopedics. 2002; 25:5 Suppl. s591–s595.4. Boden SD, Martin GJ Jr, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine (Phila Pa 1976). 1999; 24(4):320–327.5. Lee JH, Chang BS, Jeung UO, Park KW, Kim MS, Lee CK. The first clinical trial of beta-calcium pyrophosphate as a novel bone graft extender in instrumented posterolateral lumbar fusion. Clin Orthop Surg. 2011; 3(3):238–244.6. Lee JH, Chang BS, Ryu HS, Lee CK. A 90-day subchronic toxicity study of beta-calcium pyrophosphate in rat. Drug Chem Toxicol. 2009; 32(3):277–282.7. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004; 4(5):527–538.8. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976). 2002; 27(23):2662–2673.9. Garrett MP, Kakarla UK, Porter RW, Sonntag VK. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery. 2010; 66(6):1044–1049.10. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011; 11(6):471–491.11. Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011; 11(6):511–516.12. Lee JH, Lee KM, Baek HR, Jang SJ, Lee JH, Ryu HS. Combined effects of porous hydroxyapatite and demineralized bone matrix on bone induction: in vitro and in vivo study using a nude rat model. Biomed Mater. 2011; 6(1):015008.13. Ha NS, Tran TT, Tran PH, Park JB, Lee BJ. Dissolution-enhancing mechanism of alkalizers in poloxamer-based solid dispersions and physical mixtures containing poorly water-soluble valsartan. Chem Pharm Bull (Tokyo). 2011; 59(7):844–850.14. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006; 23(12):2709–2728.15. Yu CH, Lee JH, Baek HR, Nam H. The effectiveness of poloxamer 407-based new anti-adhesive material in a laminectomy model in rats. Eur Spine J. 2012; 21(5):971–979.16. Qiu QQ, Shih MS, Stock K, et al. Evaluation of DBM/AM composite as a graft substitute for posterolateral lumbar fusion. J Biomed Mater Res B Appl Biomater. 2007; 82(1):239–245.17. Bae H, Zhao L, Zhu D, Kanim LE, Wang JC, Delamarter RB. Variability across ten production lots of a single demineralized bone matrix product. J Bone Joint Surg Am. 2010; 92(2):427–435.18. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006; 31(12):1299–1306.19. Lee JH, Jang SJ, Koo TY, et al. Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. Tissue Eng Regen Med. 2011; 8(1):8–15.20. Lin X, Pena LA, Zamora PO, Campion SL, Takahashi K. Augmentation of demineralized bone matrix (DBM) mineralization by a synthetic growth factor mimetic. J Orthop Res. 2006; 24(11):2051–2058.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Fate of Demineralized Human Osteosarcoma in Rat's Muscle Pouch
- The Development of Injectable Intraocular Lens Using Thermosensitive Polymeric Hydrogel in Vitro and in Vivo
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- A Comparative Study on Regeneration of Bone Defects after the Grafts of Demineralized Bone Matrix and Hydroxyapatite
- Reactive Oxygen Species Scavenging Hydrogel Regulates Stem Cell Behavior and Promotes Bone Healing in Osteoporosis