J Korean Med Sci.
2014 Sep;29(9):1240-1246. 10.3346/jkms.2014.29.9.1240.
Clarithromycin-Based Standard Triple Therapy Can Still Be Effective for Helicobacter pylori Eradication in Some Parts of the Korea
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. jgkimd@cau.ac.kr
- KMID: 1794602
- DOI: http://doi.org/10.3346/jkms.2014.29.9.1240
Abstract
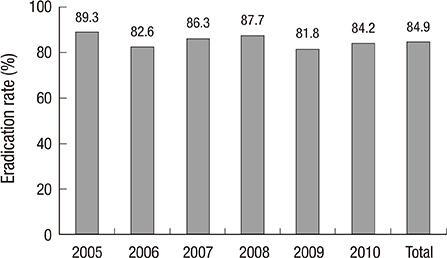
- We evaluated the antibiotic resistance rates and eradication rates of clarithromycin based triple therapy from 2005 to 2010 retrospectively. In addition, we investigated the mechanism of clarithromycin resistance in Helicobacter pylori strains isolated from Korean patients. Two hundred and twelve strains of H. pylori were isolated from 204 patients. H. pylori ATCC 43504 was used as the standard strain. The eradication rates of H. pylori from 2005 to 2010 were 89.3%, 82.6%, 86.3%, 87.7%, 81.8%, and 84.2%, respectively. Total eradication rate was 84.9%. DNA sequences of the 23S RNA gene in clarithromycin-resistant strains were determined. The resistance rates of H. pylori to amoxicillin, clarithromycin, metronidazole, tetracycline, ciprofloxacin, moxifloxacin, and levofloxacin were 9.0%, 8.5%, 36.3%, 0%, 14.2%, 14.2%, and 14.2%, respectively. The multidrug resistance rate of H. pylori was 16.5%. Sequence analysis of clarithromycin-resistant strains showed an A2144G mutation in 8 of 14 strains (57.1%), a T2183C mutation in 5 of 14 strains (35.7%), and double mutations of both A2144G and T2183C in 1 of 14 strains (7.1%). In the present study, triple therapy may still be an effective eradication therapy for H. pylori infections in Korea. The A2144G and T2183C mutations are mainly present in clarithromycin-resistant isolates.
MeSH Terms
-
Adult
Aged
Anti-Bacterial Agents/pharmacology/*therapeutic use
Asian Continental Ancestry Group
Clarithromycin/*therapeutic use
DNA, Bacterial/analysis
Drug Resistance, Bacterial/genetics
Female
Helicobacter Infections/*drug therapy/microbiology
Helicobacter pylori/drug effects/genetics/*isolation & purification
Humans
Male
Microbial Sensitivity Tests
Middle Aged
Mutation
Polymerase Chain Reaction
RNA, Ribosomal, 23S/genetics
Republic of Korea
Retrospective Studies
Sequence Analysis, DNA
Anti-Bacterial Agents
Clarithromycin
DNA, Bacterial
RNA, Ribosomal, 23S
Figure
Reference
-
1. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983; 1:1273–1275.2. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease: NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994; 272:65–69.3. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.4. Cheon JH, Kim N, Lee DH, Kim JW, Hwang JH, Park YS, Kim JM, Suh SO, Jung HC, Song IS. Trial of moxifloxacin-containing triple therapy after initial and second-line treatment failures for Helicobacter pylori infection. Korean J Gastroenterol. 2005; 45:111–117.5. Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000; 45:68–76.6. Matsuoka M, Yoshida Y, Hayakawa K, Fukuchi S, Sugano K. Simultaneous colonisation of Helicobacter pylori with and without mutations in the 23S rRNA gene in patients with no history of clarithromycin exposure. Gut. 1999; 45:503–507.7. Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Virulence factors of Helicobacter pylori in Korean isolates do not influence proinflammatory cytokine gene expression and apoptosis in human gastric epithelial cells, nor do these factors influence the clinical outcome. J Gastroenterol. 2000; 35:898–906.8. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. accessed on 1 January 2014. Available at http://www.microbiolab-bg.com/CLSI.pdf.9. Kim JM, Kim JS, Kim N, Jung HC, Song IS. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother. 2005; 56:965–967.10. Kim SJ, Kim JG, Jung K, Hong YH, Kim JH, Jung HR, Kwon JH, Yang YH, Kim HJ, Do JH, et al. Antimicrobial resistance rate of Helicobacter pylori isolates and detection of mechanism of clarithromycin resistance. Korean J Med. 2001; 61:470–478.11. Kato S, Fujimura S, Udagawa H, Shimizu T, Maisawa S, Ozawa K, Iinuma K. Antibiotic resistance of Helicobacter pylori strains in Japanese children. J Clin Microbiol. 2002; 40:649–653.12. Kim JY, Kim N, Kim SJ, Baik GH, Kim GH, Kim JM, Nam RH, Kim HB, Lee DH, Jung HC, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011; 57:221–229.13. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.14. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56:772–781.15. Kim JM, Kim JS, Jung HC, Kim N, Song IS. Antibiotic resistance of Helicobacter pylori isolated from Korean patients in 2003. Korean J Gastroenterol. 2004; 44:126–135.16. Mégraud F. Helicobacter pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004; 53:1374–1384.17. Kato M, Yamaoka Y, Kim JJ, Reddy R, Asaka M, Kashima K, Osato MS, El-Zaatari FA, Graham DY, Kwon DH. Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob Agents Chemother. 2000; 44:2214–2216.18. Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, Jung HC, Song IS. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006; 40:683–687.19. Choi YS, Cheon JH, Lee JY, Kim SG, Kim JS, Kim N, Lee DH, Kim JM, Jung HC, Song IS. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006; 48:156–161.20. Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim do H, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.21. Chung WC, Lee KM, Paik CN, Lee JR, Jung SH, Kim JD, Han SW, Chung IS. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009; 53:221–227.22. Kim JY, Kim N, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Primary antibiotic resistance of Helicobacter pylori strains and eradication rate according to gastroduodenal disease in Korea. Korean J Gastroenterol. 2011; 58:74–81.23. Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, Tanaka SK, Graham DY, Go MF. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996; 40:477–480.24. Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar CM, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997; 41:2724–2728.25. García-Arata MI, Baquero F, de Rafael L, Martín de Argila C, Gisbert JP, Bermejo F, Boixeda D, Cantón R. Mutations in 23S rRNA in Helicobacter pylori conferring resistance to erythromycin do not always confer resistance to clarithromycin. Antimicrob Agents Chemother. 1999; 43:374–376.26. Fontana C, Favaro M, Minelli S, Criscuolo AA, Pietroiusti A, Galante A, Favalli C. New site of modification of 23S rRNA associated with clarithromycin resistance of Helicobacter pylori clinical isolates. Antimicrob Agents Chemother. 2002; 46:3765–3769.27. Khan R, Nahar S, Sultana J, Ahmad MM, Rahman M. T2182C mutation in 23S rRNA is associated with clarithromycin resistance in Helicobacter pylori isolates obtained in Bangladesh. Antimicrob Agents Chemother. 2004; 48:3567–3569.28. Hultén K, Gibreel A, Sköld O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997; 41:2550–2553.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance
- Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment
- Ten Years Retrospective Study about Helicobacter pylori Eradication Rate
- Changes in the Eradication Rate of Conventional Triple Therapy for Helicobacter pylori Infection in Korea
- New Helicobacter pylori Eradication Therapies