A Simple and Efficient Multiplex PCR Assay for the Identification of Mycobacterium Genus and Mycobacterium tuberculosis Complex to the Species Level
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Health Sciences, Yonsei University, Wonju, Korea. hyelee@yonsei.ac.kr
- 2Department of Clinical Laboratory Science, College of Health Sciences, Catholic University of Pusan, Busan, Korea.
- 3Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1793171
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1220
Abstract
- PURPOSE
The Mycobacterium tuberculosis complex comprises M. tuberculosis, M. bovis, M. bovis bacillus Calmette-Guerin (BCG) and M. africanum, and causes tuberculosis in humans and animals. Identification of Mycobacterium spp. and M. tuberculosis complex to the species level is important for practical use in microbiological laboratories, in addition to optimal treatment and public health.
MATERIALS AND METHODS
A novel multiplex PCR assay targeting a conserved rpoB sequence in Mycobacteria spp., as well as regions of difference (RD) 1 and RD8, was developed and evaluated using 37 reference strains and 178 clinical isolates.
RESULTS
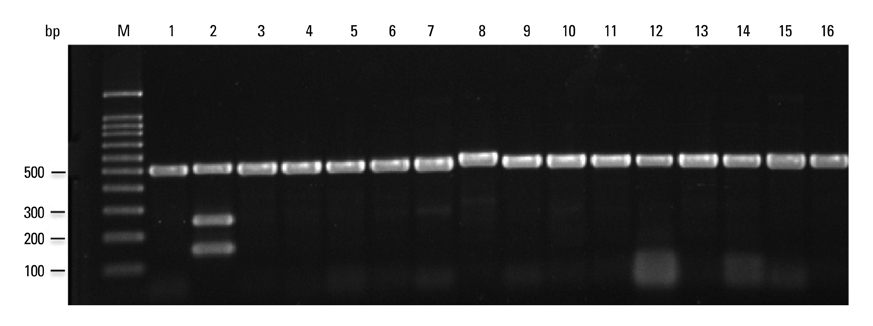
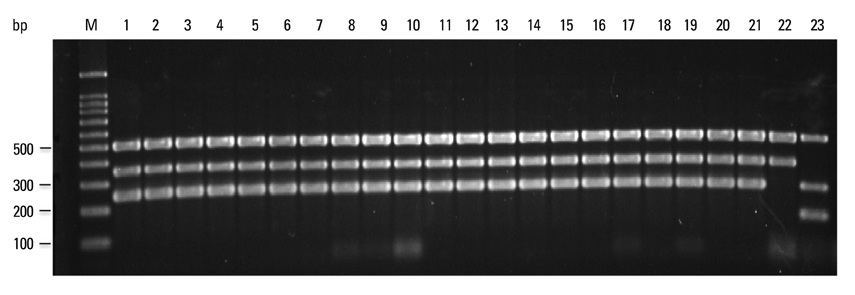
All mycobacterial strains produced a 518-bp product (rpoB), while other bacteria produced no product. Virulent M. tuberculosis complex strains, M. tuberculosis, M. bovis and M. africanum, produced a 254-bp product (RD1), while M. bovis BCG, M. microti and nontuberculous mycobacteria produced no RD1 region product. Additionally, M. tuberculosis and M. africanum produced a 150-bp product (RD8), while M. bovis and M. bovis BCG produced a 360-bp product (deleted form of RD8). M. microti and nontuberculous mycobacteria produced no RD8 region product. This assay identified all Mycobacterium spp. and all M. tuberculosis complex strains to the species level.
CONCLUSION
The multiplex PCR assay of the present study could be implemented as a routine test in microbiology laboratories, and may contribute to more effective treatment and surveillance of tuberculosis stemming from the M. tuberculosis complex.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Comparative Evaluation of Several Gene Targets for Designing a Multiplex-PCR for an Early Diagnosis of Extrapulmonary Tuberculosis
Ankush Raj, Netrapal Singh, Krishna B. Gupta, Dhruva Chaudhary, Aparna Yadav, Anil Chaudhary, Kshitij Agarwal, Mandira Varma-Basil, Rajendra Prasad, Gopal K. Khuller, Promod K. Mehta
Yonsei Med J. 2016;57(1):88-96. doi: 10.3349/ymj.2016.57.1.88.Performance of the SD Bioline TB Ag MPT64 Rapid test for quick confirmation of Mycobacterium bovis isolates from animals
Hyeon-Seop Byeon, Mi Jung Ji, Shin-Seok Kang, Sang Woo Kim, Seung-Cheol Kim, Song-Yong Park, Geehyuk Kim, Jiro Kim, Jang-Eun Cho, Bok Kyung Ku, Jae-Myung Kim, Bo-Young Jeon
J Vet Sci. 2015;16(1):31-35. doi: 10.4142/jvs.2015.16.1.31.Mycobacterium bovis infection in a wild sow (Sus scrofa): the first case in Korea
Bok Kyung Ku, Bo-Young Jeon, Jae Myung Kim, Young-Boo Jang, Yunho Jang, So Yoon Yu, Jiro Kim, Oun Kyung Moon, Suk Chan Jung, Min Kwon Lee, Tae Nam Jeong
J Vet Sci. 2016;17(3):427-429. doi: 10.4142/jvs.2016.17.3.427.
Reference
-
1. Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012; 379:1902–1913.
Article2. Ernst JD, Trevejo-Nuñez G, Banaiee N. Genomics and the evolution, pathogenesis, and diagnosis of tuberculosis. J Clin Invest. 2007; 117:1738–1745.
Article3. de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb). 2006; 86:77–109.
Article4. Lamm DL, Stogdill VD, Stogdill BJ, Crispen RG. Complications of bacillus Calmette-Guerin immunotherapy in 1,278 patients with bladder cancer. J Urol. 1986; 135:272–274.
Article5. Kamphuis JT, Buiting AG, Miseré JF, van Berge Henegouwen DP, van Soolingen D, Rensma PL. BCG immunotherapy: be cautious of granulomas. Disseminated BCG infection and mycotic aneurysm as late complications of intravesical BCG instillations. Neth J Med. 2001; 58:71–75.
Article6. O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995; 76:Suppl 1. 1–46.7. Sankar S, Ramamurthy M, Nandagopal B, Sridharan G. An appraisal of PCR-based technology in the detection of Mycobacterium tuberculosis. Mol Diagn Ther. 2011; 15:1–11.
Article8. Frothingham R. Differentiation of strains in Mycobacterium tuberculosis complex by DNA sequence polymorphisms, including rapid identification of M. bovis BCG. J Clin Microbiol. 1995; 33:840–844.
Article9. Kjeldsen MK, Bek D, Rasmussen EM, Priemé A, Thomsen VØ. Line probe assay for differentiation within Mycobacterium tuberculosis complex. Evaluation on clinical specimens and isolates including Mycobacterium pinnipedii. Scand J Infect Dis. 2009; 41:635–641.
Article10. Parsons LM, Brosch R, Cole ST, Somoskövi A, Loder A, Bretzel G, et al. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol. 2002; 40:2339–2345.
Article11. Halse TA, Escuyer VE, Musser KA. Evaluation of a single-tube multiplex real-time PCR for differentiation of members of the Mycobacterium tuberculosis complex in clinical specimens. J Clin Microbiol. 2011; 49:2562–2567.
Article12. Pinsky BA, Banaei N. Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J Clin Microbiol. 2008; 46:2241–2246.
Article13. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000; 38:2966–2971.
Article14. Gordon SV, Eiglmeier K, Garnier T, Brosch R, Parkhill J, Barrell B, et al. Genomics of Mycobacterium bovis. Tuberculosis (Edinb). 2001; 81:157–163.
Article15. Dobner P, Feldmann K, Rifai M, Löscher T, Rinder H. Rapid identification of mycobacterial species by PCR amplification of hypervariable 16S rRNA gene promoter region. J Clin Microbiol. 1996; 34:866–869.
Article16. Frota CC, Hunt DM, Buxton RS, Rickman L, Hinds J, Kremer K, et al. Genome structure in the vole bacillus, Mycobacterium microti, a member of the Mycobacterium tuberculosis complex with a low virulence for humans. Microbiology. 2004; 150(Pt 5):1519–1527.
Article17. Vasconcellos SE, Huard RC, Niemann S, Kremer K, Santos AR, Suffys PN, et al. Distinct genotypic profiles of the two major clades of Mycobacterium africanum. BMC Infect Dis. 2010; 10:80.
Article18. Butler WR, Jost KC Jr, Kilburn JO. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991; 29:2468–2472.
Article19. Streicher EM, Victor TC, van der Spuy G, Sola C, Rastogi N, van Helden PD, et al. Spoligotype signatures in the Mycobacterium tuberculosis complex. J Clin Microbiol. 2007; 45:237–240.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "A Simple and Efficient Multiplex PCR Assay for the Identification of Mycobacterium Genus and Mycobacterium tuberculosis Complex to the Species Level" by Kim Y, et al. (Yonsei Med J 2013;54:1220-6.)
- Identification of Mycobacterium Species by Multiplex PCR-Restriction Fragment Length Polymorphism Assay
- Multiplex PCR Assay for Identification of Mycobacterial Species Isolated from Liquid Cultures
- Identification of Non-tuberculosous Mycobacteria by HaeIII Restriction Enzyme Analysis
- Identification of Mycobacterium avium complex ( MAC ) clinical Strains to a Species Level by Sequencing and PCR - SSCP Analysis of rpoB DNA