Ann Lab Med.
2014 Sep;34(5):345-353. 10.3343/alm.2014.34.5.345.
Proteomic Profiling of Serum from Patients with Tuberculosis
- Affiliations
-
- 1Clinical Proteomics Laboratory, Seoul National University Hospital, Seoul, Korea.
- 2Biomedical Research Institute and Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jhlee7@snubh.org
- KMID: 1791948
- DOI: http://doi.org/10.3343/alm.2014.34.5.345
Abstract
- BACKGROUND
Effective treatment and monitoring of tuberculosis (TB) requires biomarkers that can be easily evaluated in blood samples. The aim of this study was to analyze the serum proteome of patients with TB and to identify protein biomarkers for TB.
METHODS
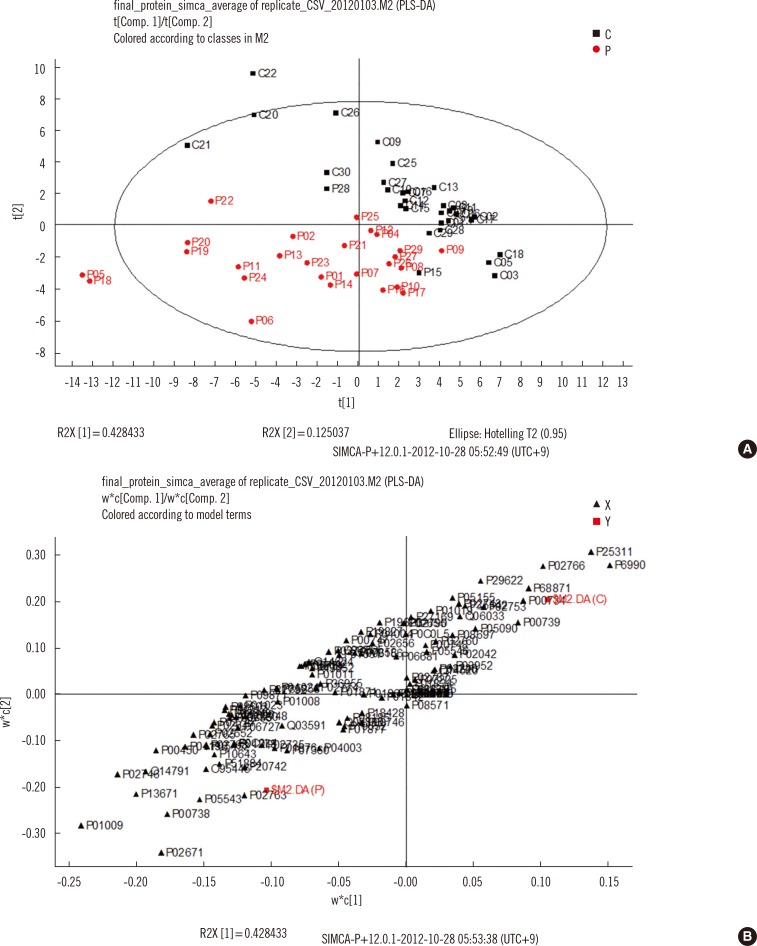
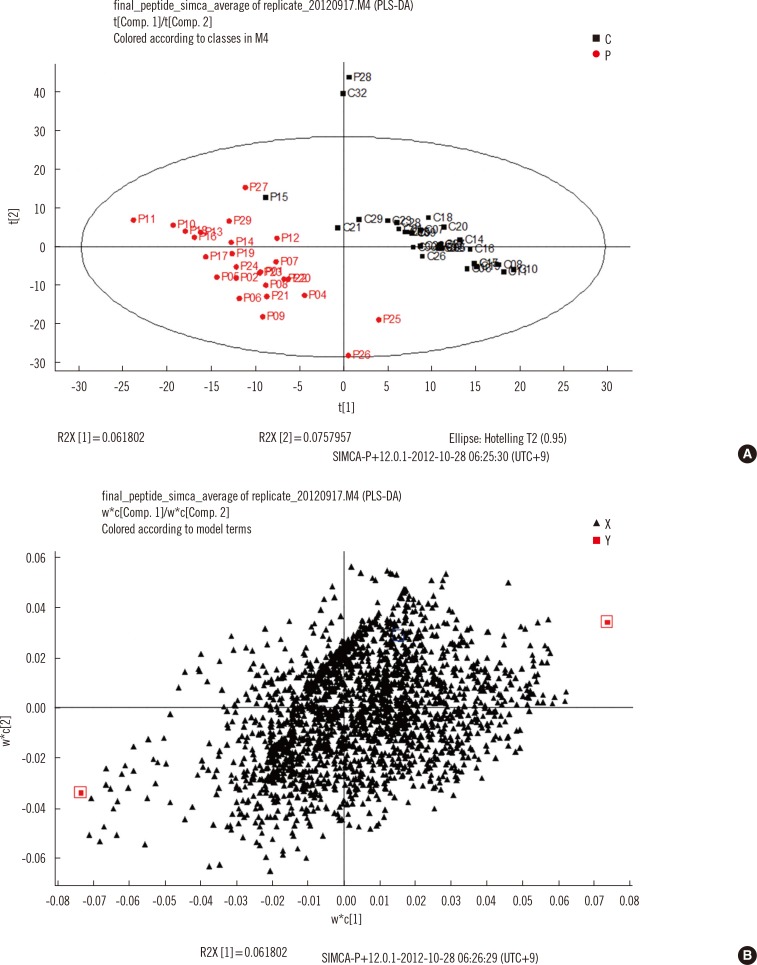
Serum samples from 26 TB patients and 31 controls were analyzed by using nano-flow ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry in data-independent mode, and protein and peptide amounts were calculated by using a label-free quantitative approach. The generated data were analyzed by using principal component analysis and partial least squares discriminant analysis, a multivariate statistical method.
RESULTS
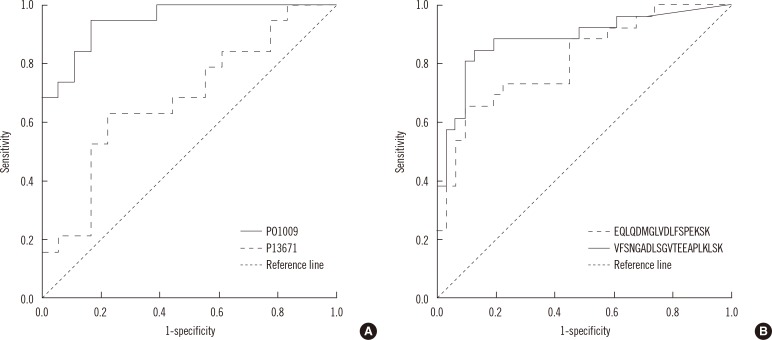
Of more than 500 proteins identified, alpha-1-antitrypsin was the most discriminative, which was 4.4 times higher in TB patients than in controls. Peptides from alpha-1-antitrypsin and antithrombin III increased in TB patients and showed a high variable importance in the projection scores and coefficient in partial least square discriminant analysis.
CONCLUSIONS
Sera from patients with TB had higher alpha-1-antitrypsin levels than sera from control participants. Alpha-1-antitrypsin levels may aid in the diagnosis of TB.
Keyword
MeSH Terms
-
Adult
Aged
Antithrombin III/analysis
Biological Markers/blood
Chromatography, High Pressure Liquid
Discriminant Analysis
Female
Humans
Male
Middle Aged
Multivariate Analysis
Proteome/*analysis
*Proteomics
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Tuberculosis/*blood/genetics/metabolism
alpha 1-Antitrypsin/analysis
Antithrombin III
Biological Markers
Proteome
alpha 1-Antitrypsin
Figure
Reference
-
1. Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992; 5:1–25.
Article2. Neonakis IK, Gitti Z, Krambovitis E, Spandidos DA. Molecular diagnostic tools in mycobacteriology. J Microbiol Methods. 2008; 75:1–11.
Article3. Zhang Z, Chan DW. The road from discovery to clinical diagnostics: lessons learned from the first FDA-cleared in vitro diagnostic multivariate index assay of proteomic biomarkers. Cancer Epidemiol Biomarkers Prev. 2010; 19:2995–2999.4. Boja ES, Rodriguez H. The path to clinical proteomics research: integration of proteomics, genomics, clinical laboratory and regulatory science. Korean J Lab Med. 2011; 31:61–71.
Article5. Cheng FY, Blackburn K, Lin YM, Goshe MB, Williamson JD. Absolute protein quantification by LC/MS(E) for global analysis of salicylic acid-induced plant protein secretion responses. J Proteome Res. 2009; 8:82–93.
Article6. Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010; 375:1920–1937.
Article7. Agranoff D, Fernandez-Reyes D, Papadopoulos MC, Rojas SA, Herbster M, Loosemore A, et al. Identification of diagnostic markers for tuberculosis by proteomic fingerprinting of serum. Lancet. 2006; 368:1012–1021.
Article8. Wong CT, Saha N. Changes in serum proteins (albumin, immunoglobulins and acute phase proteins) in pulmonary tuberculosis during therapy. Tubercle. 1990; 71:193–197.
Article9. Emmett M, Miller JL, Crowle AJ. Protein abnormalities in adult respiratory distress syndrome, tuberculosis, and cystic fibrosis sera. Proc Soc Exp Biol Med. 1987; 184:74–82.
Article10. Grange JM, Kardjito T, Setiabudi I. A study of acute-phase reactant proteins in Indonesian patients with pulmonary tuberculosis. Tubercle. 1984; 65:23–39.
Article11. Schaller J, Gerber S, editors. Human blood plasma proteins:structure and function. Chichester, West Sussex: John Wiley & Sons;2008.12. Laurell CB, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. COPD. 2013; 10(Suppl 1):3–8.
Article13. Lisowska-Myjak B. AAT as a diagnostic tool. Clin Chim Acta. 2005; 352:1–13.
Article14. Miravitlles M. Alpha-1-antitrypsin and other proteinase inhibitors. Curr Opin Pharmacol. 2012; 12:309–314.
Article15. Cylwik B, Chrostek L, Gindzienska-Sieskiewicz E, Sierakowski S, Szmitkowski M. Relationship between serum acute-phase proteins and high disease activity in patients with rheumatoid arthritis. Adv Med Sci. 2010; 55:80–85.
Article16. Wu W, Juan WC, Liang CR, Yeoh KG, So J, Chung MC. S100A9, GIF and AAT as potential combinatorial biomarkers in gastric cancer diagnosis and prognosis. Proteomics Clin Appl. 2012; 6:152–162.
Article17. Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999; 19:173–198.
Article18. Sai Baba KS, Moudgil KD, Jain RC, Srivastava LM. Complement activation in pulmonary tuberculosis. Tubercle. 1990; 71:103–107.
Article19. Turken O, Kunter E, Sezer M, Solmazgul E, Cerrahoglu K, Bozkanat E, et al. Hemostatic changes in active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002; 6:927–932.20. Robson SC, White NW, Aronson I, Woollgar R, Goodman H, Jacobs P. Acute-phase response and the hypercoagulable state in pulmonary tuberculosis. Br J Haematol. 1996; 93:943–949.
Article21. Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013; 783:225–250.
Article22. Kirwan GM, Johansson E, Kleemann R, Verheij ER, Wheelock AM, Goto S, et al. Building multivariate systems biology models. Anal Chem. 2012; 84:7064–7071.
Article23. Fan L, Zhang W, Yin M, Zhang T, Wu X, Zhang H, et al. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol. 2012; 51:473–479.
Article24. Liu Y, Sun X, Di D, Quan J, Zhang J, Yang X. A metabolic profiling analysis of symptomatic gout in human serum and urine using high performance liquid chromatography-diode array detector technique. Clin Chim Acta. 2011; 412:2132–2140.
Article25. Wang X, Zhang A, Han Y, Wang P, Sun H, Song G, et al. Urine metabolomics analysis for biomarker discovery and detection of jaundice syndrome in patients with liver disease. Mol Cell Proteomics. 2012; 11:370–380.
Article26. Li Y, Zhou K, Zhang Z, Sun L, Yang J, Zhang M, et al. Label-free quantitative proteomic analysis reveals dysfunction of complement pathway in peripheral blood of schizophrenia patients: evidence for the immune hypothesis of schizophrenia. Mol Biosyst. 2012; 8:2664–2671.
Article27. Ray S, Renu D, Srivastava R, Gollapalli K, Taur S, Jhaveri T, et al. Proteomic investigation of falciparum and vivax malaria for identification of surrogate protein markers. PLoS One. 2012; 7:e41751.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of new biomarkers of hepatic cancer stem cells through proteomic profiling
- The Proteomic Analysis of Human Placenta with Pre-eclampsia and Normal Pregnancy
- Proteomic profiling of bladder cancer for precision medicine in the clinical setting: A review for the busy urologist
- Proteomic Identification of Proteins Suggestive of Immune-Mediated Response or Neuronal Degeneration in Serum of Achalasia Patients
- Identification of Leukemia Surface Proteins Using a Proteomic Technique