Ann Lab Med.
2014 May;34(3):187-197. 10.3343/alm.2014.34.3.187.
Harmonization: the Sample, the Measurement, and the Report
- Affiliations
-
- 1Department of Pathology, Virginia Commonwealth University, Richmond, VA, USA. gmiller@vcu.edu
- 2Pathology Queensland, Department of Chemical Pathology, Royal Brisbane and Women's Hospital, Brisbane, Queensland, Australia.
- 3Blood Sciences, Old Medical School, Leeds Teaching Hospitals Trust, Leeds, UK.
- 4SydPath, Department of Chemical Pathology, St. Vincent's Hospital, Darlinghurst, NSW, and University of New South Wales, Kensington, Australia.
- KMID: 1791919
- DOI: http://doi.org/10.3343/alm.2014.34.3.187
Abstract
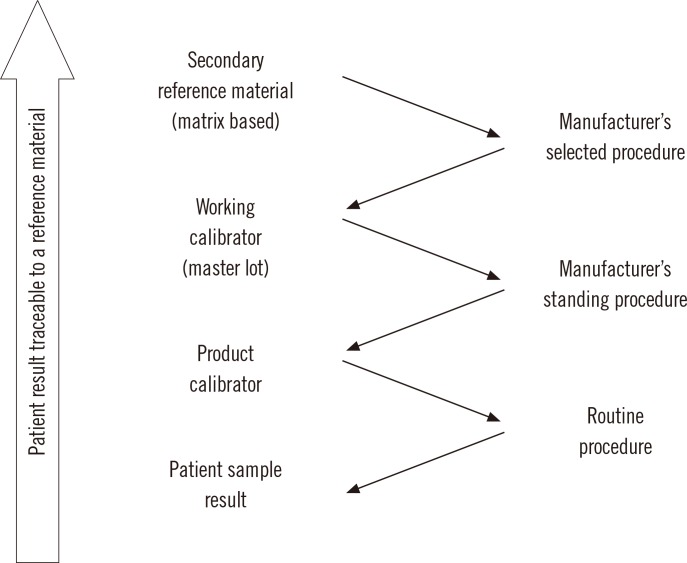
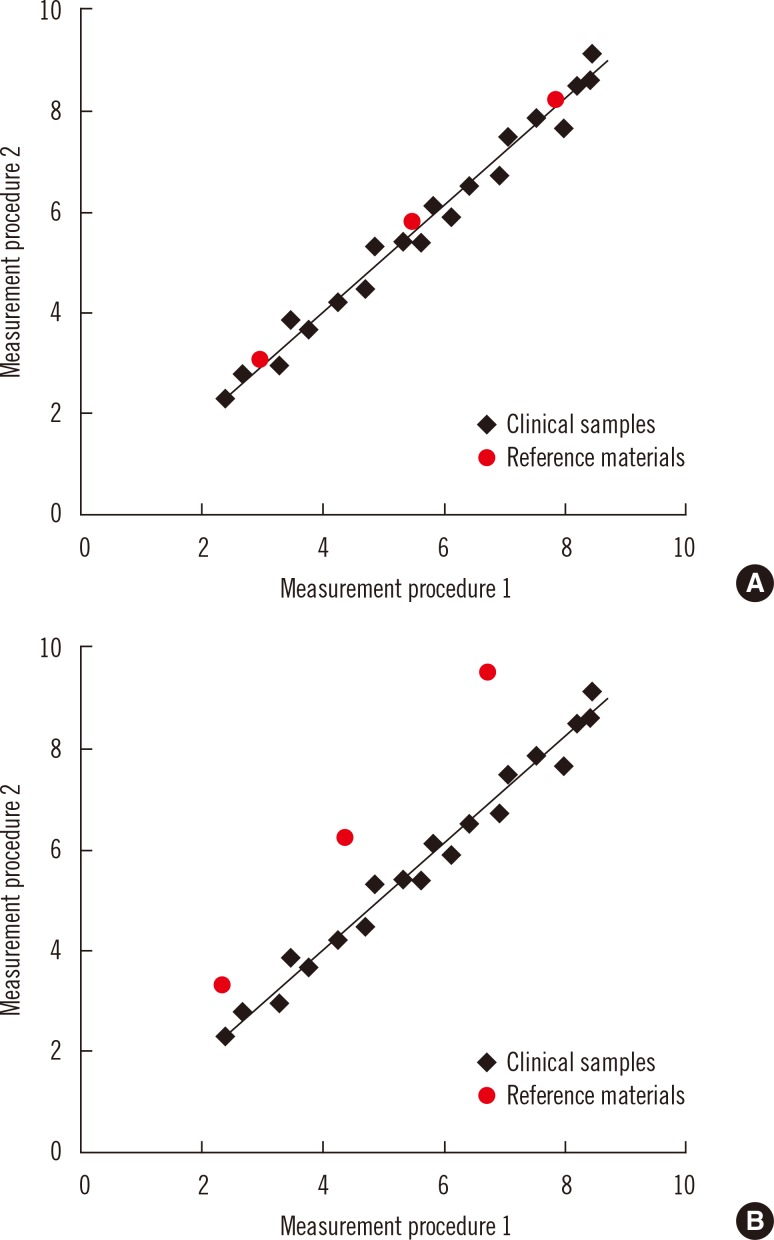
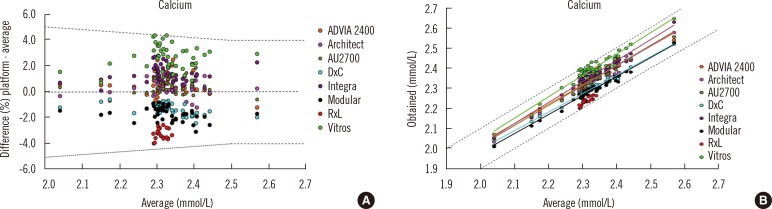
- Harmonization of clinical laboratory results means that results are comparable irrespective of the measurement procedure used and where or when a measurement was made. Harmonization of test results includes consideration of pre-analytical, analytical, and post-analytical aspects. Progress has been made in each of these aspects, but there is currently poor coordination of the effort among different professional organizations in different countries. Pre-analytical considerations include terminology for the order, instructions for preparation of the patient, collection of the samples, and handling and transportation of the samples to the laboratory. Key analytical considerations include calibration traceability to a reference system, commutability of reference materials used in a traceability scheme, and specificity of the measurement of the biomolecule of interest. International organizations addressing harmonization include the International Federation for Clinical Chemistry and Laboratory Medicine, the World Health Organization, and the recently formed International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR). The ICHCLR will provide a prioritization process for measurands and a service to coordinate global harmonization activities to avoid duplication of effort. Post-analytical considerations include nomenclature, units, significant figures, and reference intervals or decision values for results. Harmonization in all of these areas is necessary for optimal laboratory service. This review summarizes the status of harmonization in each of these areas and describes activities underway to achieve the goal of fully harmonized clinical laboratory testing.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Periodic Comparability Verification and Within-Laboratory Harmonization of Clinical Chemistry Laboratory Results at a Large Healthcare Center With Multiple Instruments
Youngwon Nam, Joon Hee Lee, Sung Min Kim, Sun-Hee Jun, Sang Hoon Song, Kyunghoon Lee, Junghan Song
Ann Lab Med. 2022;42(2):150-159. doi: 10.3343/alm.2022.42.2.150.
Reference
-
1. Sacks DB. Burtis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5th eds. Philadelphia: Elsevier;2012. p. 719.2. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011; 57:e1–e47. PMID: 21617152.
Article3. Wu AHB. Tietz clinical guide to laboratory tests. 4th ed. St. Louis: WB Saunders;2006.4. Clinical and Laboratory Standards Institute. CLSI document EP29-A. Expression of measurement uncertainty in laboratory medicine; Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute;2012.5. White GH. Basics of estimating measurement uncertainty. Clin Biochem Rev. 2008; 29(S1):S53–S60. PMID: 18852859.6. Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem. 2009; 55:1067–1075. PMID: 19359540.
Article7. ISO 17511:2003. In vitro diagnostic medical devices -measurement of quantities in biological samples-metrological traceability of values assigned to calibrators and control materials. Geneva, Switzerland: 2003 International Organization for Standardization;2003.8. Blirup-Jensen S. Protein standardization II: dry mass determination procedure for the determination of the dry mass of a pure protein preparation. Clin Chem Lab Med. 2001; 39:1090–1097. PMID: 11831624.
Article9. Zegers I, Keller T, Schreiber W, Sheldon J, Albertini R, Blirup-Jensen S, et al. Characterization of the new serum protein reference material ERM-DA470k/IFCC: value assignment by immunoassay. Clin Chem. 2010; 56:1880–1888. PMID: 20923953.
Article10. Sturgeon CM, Sprague SM, Metcalfe W. Variation in parathyroid hormone immunoassay results--a critical governance issue in the management of chronic kidney disease. Nephrol Dial Transplant. 2011; 26:3440–3445. PMID: 22039013.
Article11. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012; 126:2020–2035. PMID: 22923432.
Article12. Weycamp C, Eckfeldt J, Vesper H, Thienpont L, Burns C, Caliendo A, editors. Toolbox of technical procedures to be considered when developing a process to achieve harmonization for a measurand. Updated on Jun 2013. http://www.harmonization.net/Resource/Documents/Tool_Box_2013.pdf.13. Van Houcke SK, Van Aelst S, Van Uytfanghe K, Thienpont LM. Harmonization of immunoassays to the all-procedure trimmed mean - proof of concept by use of data from the insulin standardization project. Clin Chem Lab Med. 2013; 51:e103–e105. PMID: 23152424.
Article14. Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006; 52:553–554. PMID: 16595820.
Article15. The Joint Committee for Guides in metrology. International vocabulary of metrology-Basic and general concepts and associated terms. VIM. 3rd edn. Sèvres, France: JCGM 200;2008. accessed on Jan 2014. See http://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf.16. Miller WG, Myers GL. Commutability still matters. Clin Chem. 2013; 59:1291–1293. PMID: 23780914.
Article17. Vesper HW, Miller WG, Myers GL. Reference materials and commutability. Clin Biochem Rev. 2007; 28:139–147. PMID: 18392124.18. Fasce CF Jr, Rej R, Copeland WH, Vanderlinde RE. A discussion of enzyme reference materials: applications and specifications. Clin Chem. 1973; 19:5–9. PMID: 4683368.
Article19. Clinical and Laboratory Standards Institute. CLSI Document EP30-A. Characterization and qualification of commutable reference materials for laboratory medicine; Approved guideline. Wayne, PA: CLSI;2010.20. Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, et al. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin Chem. 1998; 44:1198–1208. PMID: 9625043.
Article21. Gillery P, Young IS. Progress toward standardization: an IFCC Scientific Division Perspective. Clin Chem Lab Med. 2013; 51:915–918. PMID: 23435099.
Article22. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Off J Eur Communities. 1998; 12. 07. L331:1–37.23. Joint Committee for Traceability in Laboratory Medicine (JCTLM). Accessed on Jan 2014. http://www.bipm.org/jctlm/.24. Greg Miller W, Myers GL, Gantzer ML, Kahn SE, Schönbrunner ER, Thienpont LM, et al. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem. 2011; 57:1108–1117. PMID: 21677092.
Article25. American Association of Clinical Chemistry (AACC). International consortium for harmonization of clinical laboratory results (ICHCLR). Updated on Aug 2013. http://www.harmonization.net/Pages/default.aspx.26. Jones R, Batstone G, Gutteridge C, Croal B, Barnes I. NLMC-more than just for pathology. Bull Roy Coll Pathol. 2012; 160:233–236.27. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–3281. PMID: 18552288.
Article28. Kilpatrick ES, Rigby AS, Atkin SL, Barth JH. Glycemic control in the 12 months following a change to SI hemoglobin A1c reporting units. Clin Chem. 2013; 59:1457–1460. PMID: 23794734.
Article29. The UCUM Organization. The Unified Code for Units of Measure. Updated on Oct 2013. http://unitsofmeasure.org.30. Pathology Harmony, UK. Accessed on Jan 2014. http://www.pathologyharmony.co.uk.31. Royal College of Pathologists of Australasia. Pathology terminology and information standardization downloads. Updated on Nov 2013. http://www.rcpa.edu.au/Library/Practising-Pathology/PTIS/APUTS-Downloads.32. International Federation of Clinical Chemistry and Laboratory Medicine, Nomenclature, Properties and Units (C-NPU) in collaboration with International Union of Pure and Applied Chemistry (IUPAC). Accessed on Jan 2014. http://www.ifcc.org/ifcc-scientific-division/sd-committees/c-npu/.33. Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med. 2013; 51:741–751. PMID: 23435100.
Article34. Jones GR, Barker A, Tate J, Lim CF, Robertson K. The case for common reference intervals. Clin Biochem Rev. 2004; 25:99–104. PMID: 18458709.35. Jassam N, Yundt-Pacheco J, Jansen R, Thomas A, Barth JH. Can current analytical quality performance of UK clinical laboratories support evidence-based guidelines for diabetes and ischaemic heart disease? --A pilot study and a proposal. Clin Chem Lab Med. 2013; 51:1579–1584. PMID: 23525878.36. Jones GR. Laboratory reporting of urine protein and albumin. Clin Biochem Rev. 2011; 32:103–107. PMID: 21611084.37. Sikaris K. Application of the Stockholm hierarchy to defining the quality of reference intervals and clinical decision limits. Clin Biochem Rev. 2012; 33:141–148. PMID: 23267246.38. Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Mårtensson A, et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest. 2004; 64:271–284. PMID: 15223694.
Article39. Ozarda Y, Ichihara K, Barth JH, Klee G. Committee on Reference Intervals and Decision Limits (C-RIDL), International Federation for Clinical Chemistry and Laboratory Medicine. Protocol and standard operating procedures for common use in a worldwide multicenter study on reference values. Clin Chem Lab Med. 2013; 51:1027–1040. PMID: 23633469.
Article40. Berg J, Lane V. Pathology Harmony; a pragmatic and scientific approach to unfounded variation in the clinical laboratory. Ann Clin Biochem. 2011; 48:195–197. PMID: 21555538.
Article41. Australasian Association of Clinical Biochemists. Australasian Association of Clinical Biochemists Harmonisation Activity. Accessed on Jan 2014. www.aacb.asn.au/professionaldevelopment/harmonisation.42. Koerbin G, Sikaris KA, Jones GR, Ryan J, Reed M, Tate J. On behalf of the AACB Committee for Common Reference Intervals. Evidence-based approach to harmonized reference intervals. Clin Chim Acta. 2013; doi: 10.1016/j.cca.2013.10.021. [Epub ahead of print].43. Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008; 29(S1):S93–S97. PMID: 18852866.44. Jones GR, Sikaris K, Gill J. 'Allowable Limits of Performance' for External Quality Assurance Programs - an Approach to Application of the Stockholm Criteria by the RCPA Quality Assurance Programs. Clin Biochem Rev. 2012; 33:133–139. PMID: 23267245.45. Allowable limits of performance for the Royal College of Pathologists of Australasia Quality Assurance Program. available at: http://www.rcpaqap.com.au/wp-content/uploads/2013/06/chempath/Allowable%20Limits%20of%20Performance.pdf.46. Jones GR. Validating common reference intervals in routine laboratories. Clin Chim Acta. 2013; doi: 10.1016/j.cca.2013.10.005. [Epub ahead of print].
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translation: Roadmap for Harmonization of Clinical Laboratory Measurement Procedures
- Quantitative Evaluation of the Real-World Harmonization Status of Laboratory Test Items Using External Quality Assessment Data
- Harmonization of Thyroid-Stimulating Hormone and Thyroid Hormone Measurements Using Recalibration via Percentile Transformation
- Harmonization of laboratory results by data adjustment in multicenter clinical trials
- An Accurate Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry Method for Serum C-Peptide and Its Use in Harmonization in China