J Korean Med Sci.
2004 Jun;19(3):390-396. 10.3346/jkms.2004.19.3.390.
HER-2/neu Oncogene Amplification by Chromogenic in situ Hybridization in 130 Breast Cancers Using Tissue Microarray and Clinical Follow-up Studies
- Affiliations
-
- 1Department of Clinical Pathology, The Catholic University of Korea, College of Medicine, Seoul, Korea. ejlpath@catholic.ac.kr
- 2Department of General Surgery, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- KMID: 1786817
- DOI: http://doi.org/10.3346/jkms.2004.19.3.390
Abstract
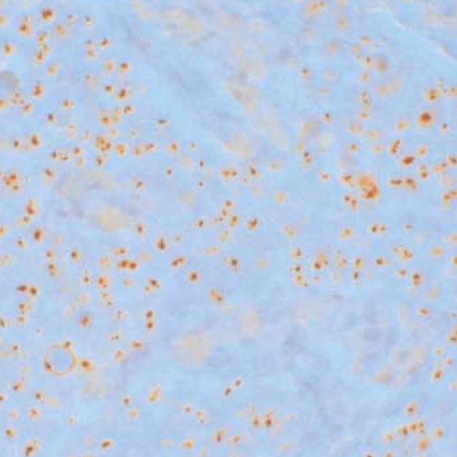
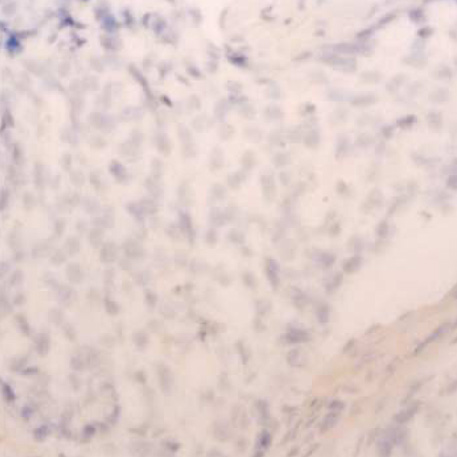
- Determining of HER-2/neu oncogene amplification has become clinically important for managing breast cancer. Fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) are currently regarded as the standard methods. Chromogenic in situ hybridization (CISH) was investigated as a new modification with an accurate, sensitive technique. From 1998 to 2002, using CISH and IHC, the amplification and protein expression of the HER-2/neu oncogene were examined using paraffin sections in 130 breast carcinomas and to determine the prognostic role of HER-2/neu for outcome after a follow-up of 24- 64 months. Amplifications by CISH and overexpression by IHC were observed in 28 (22%) and 27 cases (20.8%), respectively. Of the 104 patients, 20 patients (19.2%) with amplification had a shorter disease-free interval (34.9 months vs. 38.0 months in controls) (p=0.372). 15 patients (14.4%) had a disease recurrence, but there is no significant difference between 3 patients amplifying the oncogene and 12 patients without oncogene (20.6 months vs. 19.6 months) (p=0.862). 6 patients (5.8%) of these died. CISH is a useful alternative, particularly for confirming the IHC results. There is no relationship between the early recurrence and the HER-2/neu positive group, but lymph node status was statistically significant.
Keyword
MeSH Terms
-
Adult
Aged
Breast Neoplasms/*genetics/metabolism/mortality
Disease-Free Survival
Female
Follow-Up Studies
Genes, erbB-2/*genetics
Human
Immunohistochemistry
In Situ Hybridization
In Situ Hybridization, Fluorescence
Lymphatic Metastasis
Middle Aged
*Oligonucleotide Array Sequence Analysis
Prognosis
Protein Array Analysis
Receptor, erbB-2/biosynthesis
Sensitivity and Specificity
Support, Non-U.S. Gov't
Treatment Outcome
Figure
Reference
-
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.
Article2. Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002. 20:719–726.
Article3. Tanner M, Jarvinen P, Isola J. Amplification of HER-2/neu and Topoisomerase IIα in primary and metastatic breast cancer. Cancer Res. 2001. 61:5345–5348.4. Riou G, Mathieu MC, Barrois M, Le Bihan ML, Ahomadegbe JC, Benard J, Le MG. c-erbB-2 (HER-2/neu) gene amplification is a better indicator of poor prognosis than protein over-expression in operable breast cancer patients. Int J Cancer. 2001. 95:266–270.5. Yamauchi H, Stearns V, Hayes DF. When is a tumor marker ready for prime time? A case study of c-erbB-2 as a predictive factor in breast cancer. J Clin Oncol. 2001. 19:2334–2356.
Article6. Carr JA, Havstad S, Zarbo RJ, Divine G, Mackowiak P, Velanovich V. The association of HER-2/neu amplification with breast cancer recurrence. Arch Surg. 2000. 135:1469–1474.
Article7. Ross JS, Fletcher JA. HER-2/neu (c-erbB-2) gene and protein in breast cancer. Am J Clin Pathol. 1999. 112:Suppl 1. S53–S67.8. Gupta D, Middleton LP, Whitaker MJ, Abrams J. Comparison of fluorescence and chromogenic in situ hybridization for detection of HER-2/neu oncogene in breast cancer. Am J Clin Pathol. 2003. 119:381–387.
Article9. Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivot trials. Oncology. 2001. 61:Suppl 2. 14–21.10. Pegram MD, Konecny G, Slamon DJ. The molecular and cellular biology of HER-2/gene amplification/overexpression and the clinical development of Herceptin (trastuzumab) therapy for breast cancer. Cancer Treat Res. 2000. 103:57–75.11. Ross JS, Gray K, Gray GS, Worland PJ, Rolfe M. Anticancer antibodies. Am J Clin Pathol. 2003. 119:472–485.
Article12. Slamon D, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER-2 for metastatic breast cancer that overexpresses HER-2. N Engl J Med. 2001. 344:783–792.
Article13. Rhodes A, Jasani B, Anderson E, Dodson AR, Balaton AJ. Evaluation of HER-2/neu immunohistochemical assay sensitivity and scoring on formalin-fixed and paraffin-processed cell lines and breast tumors. A comparative study involving results from laboratories in 21 countries. Am J Clin Pathol. 2002. 118:408–417.14. Barnes DM, Lammie GA, Millis RR, Gullick WL, Allen DS, Altman DG. An immunohistochemical evaluation of c-erbB-2 expression in human breast carcinoma. Br J Cancer. 1988. 58:448–452.
Article15. Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, Isola J. Chromogenic in situ hybridization. A practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000. 157:1467–1472.16. Tubbs R, Skacel M, Pettay J, Powell R, Myles J, Hicks D, Sreenan J, Roche P, Stoler MH, Hainfeld J. Interobserver interpretative reproducibility of GOLDFISH, a first generation gold-facilitated autometallographic bright field in situ hybridization assay for HER-2/neu amplification in invasive mammary carcinoma. Am J Surg Pathol. 2002. 26:908–913.
Article17. Kumamoto H, Sasano H, Taniguchi T, Suzuki T, Moriya T, Ichinohasama R. Chromogenic in situ hybridization analysis of HER-2/neu status in breast carcinoma: application in screening of patients for trastuzumab (Herceptin) therapy. Pathol Int. 2001. 51:579–584.18. Dandachi N, Dietze O, Hauser-Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER-2 oncogene in archival human breast carcinoma. Lab Invest. 2002. 82:1007–1014.
Article19. Zhao J, Wu R, Au A, Marquez A, Yu Y, Shi Z. Determination of HER-2/neu gene amplification by chromogenic in situ hybridization (CISH) in archival breast carcinoma. Mod Pathol. 2002. 15:657–665.20. Bast RC, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001. 19:1865–1878.
Article21. Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, Liu CL, van de Rijn M, Gilks CB. Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol. 2002. 15:1374–1380.
Article22. Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES. Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol. 2003. 16:79–84.
Article23. van de Vijver M. Emerging technologies for HER 2 testing. Oncology. 2002. 63:Suppl 1. 33–38.24. Jacobs TW, Bown AM, Yaziji H, Barnes MJ, Shinitt SJ. Specificity of Hercep Test in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999. 17:1983–1987.25. Bilous M, Dowsett M, Hanna W, Isola J, Lebeau A, Moreno A, Penault-Llorca F, Ruschoff J, Tomasic G, van de Vijver M. Current perspectives on HER 2 testing: A review of national testing guidelines. Mod Pathol. 2003. 16:173–182.26. Jimenez RE, Wallis T, Tabasczka P, Visscher DW. Determination of HER-2/neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2000. 13:37–45.
Article27. Ridolfi RL, Jamehdor MR, Arber JM. HER-2/neu testing in breast carcinoma : a combined immunohistochemical and fluorescence in situ hybridization approach. Mod Pathol. 2000. 13:866–873.28. Wang S, Saboorian MH, Frenkel E, Hynan L, Gokaslan ST, Ashfaq R. Laboratory assessment of the status of HER-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridization assays. J Clin Pathol. 2000. 53:374–381.29. Lee TJ, Oh HG, Kwon GY, Kim MK, Park ES, Yoo JH. HER-2/neu oncogene amplification by chromogenic in situ hybridization and immunohistochemical expression of Topoisomerase II-α in the breast cancer. Korean J Pathol. 2003. 37:26–34.30. Latta EK, Tjan S, Parkes RK, O'Malley FP. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002. 15:1318–1325.
Article31. Dolan J, Curran B, Henry K, Lindley R, Leader M. C-erbB-2 protein expression and survival in breast carcinoma [abstract]. J Pathol. 1989. 158:354A.32. Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998. 16:462–469.33. Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003. 21:46–53.
Article34. Roche PC, Ingle JN. Increased HER-2 with U.S. Food and Drug Administration-approved antibody. J Clin Oncol. 1999. 17:434.
Article35. Cell Markers and Cytogenetics Committees College of American Pathologists. Clinical laboratory assays for HER-2/neu amplification and overexpression: quality assurance, standardization, and proficiency testing. Arch Pathol Lab Med. 2002. 126:803–808.36. Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000. 18:3651–3664.
Article37. Park K, Kim J, Lim S, Han S, Lee JY. Comparing fluorescence in situ hybridization and chromogenic in situ hybridization methods to determine the HER-2/neu status in primary breast carcinoma using tissue microarray. Mod Pathol. 2003. 16:937–943.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HER-2/neu Oncogene Amplification by Chromogenic in situ Hybridization and Immunohistochemical Expression of Topoisomerase II-alpha in the Breast Cancer
- Amplification of the HER-2/neu Oncogene and Topoisomerase II-alpha by Chromogenic in Situ Hybridization in Breast Cancer
- Chromogenic In Situ Hybridization Analysis to Determinate HER-2/neu Status in Breast Carcinoma
- The Analysis of HER-2/neu Gene Status and Correlation with Other Clinico-Pathologic Factors for Breast Cancer Using Tissue Microarray
- Comparing Fluorescence In Situ Hybridization and Immunohistochemistry to Determine the HER-2/neu Status in Breast Carcinoma