J Periodontal Implant Sci.
2010 Oct;40(5):211-219. 10.5051/jpis.2010.40.5.211.
Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. guy@snu.ac.kr
- 2Craniomaxillofacial Reconstructive Science Major, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 1783561
- DOI: http://doi.org/10.5051/jpis.2010.40.5.211
Abstract
- PURPOSE
Fibronectin (FN) has been shown to stimulate bone regeneration in animal models. The aim of this study was to evaluate the capacity of bovine bone mineral coated with synthetic oligopeptides to enhance bone regeneration in rabbit calvarial defects.
METHODS
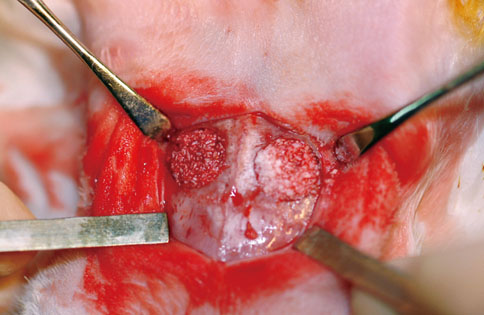
Oligopeptides including fibrin-binding sequences of FN repeats were synthesized on the basis of primary and tertiary human plasma FN structures. Peptide coated and uncoated bone minerals were implanted into 10 mm calvarial defects in New Zealand white rabbits, and the animals were sacrificed at 4 or 8 weeks after surgery. After specimens were prepared, histologic examination and histomorphometric analysis were performed.
RESULTS
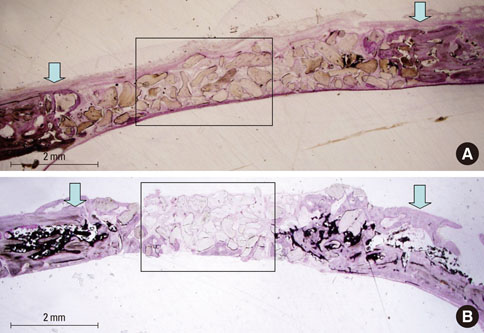
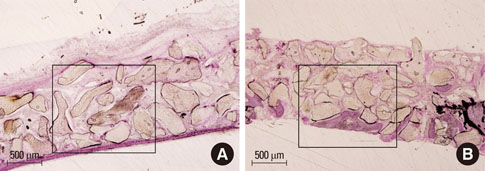
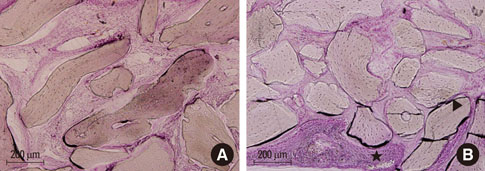
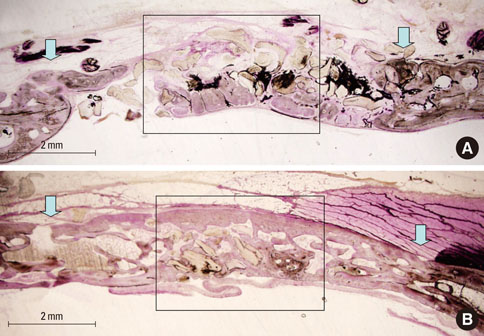
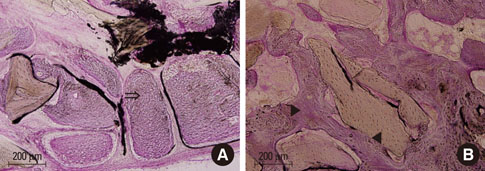
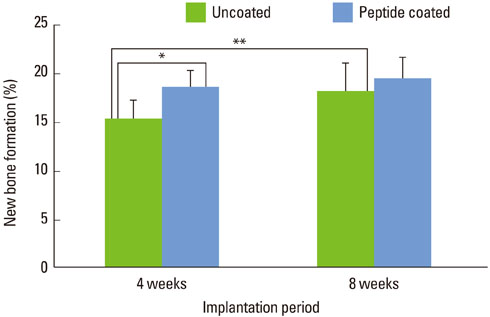
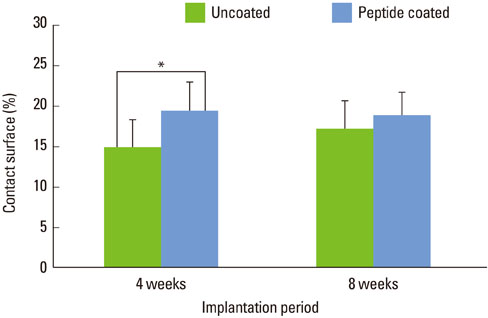
At 4 weeks after surgery, the uncoated groups showed a limited amount of osteoid formation at the periphery of the defect and the oligopeptide coated groups showed more osteoid formation and new bone formation in the center of the defect as well as at the periphery. At 8 weeks, both sites showed increased new bone formation. However, the difference between the two sites had reduced.
CONCLUSIONS
Fibrin-binding synthetic oligopeptide derived from FN on deproteinized bovine bone enhanced new bone formation in rabbit calvarial defects at the early healing stage. This result suggests that these oligopeptides can be beneficial in reconstructing oral and maxillofacial deformities or in regenerating osseous bone defects.
Keyword
MeSH Terms
Figure
Reference
-
1. Hallman M, Cederlund A, Lindskog S, Lundgren S, Sennerby L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation. Results after 6 to 8 months of healing. Clin Oral Implants Res. 2001. 12:135–143.
Article2. Carlson ER, Marx RE. Part II. Mandibular reconstruction using cancellous cellular bone grafts. J Oral Maxillofac Surg. 1996. 54:889–897.3. Petrovic L, Schlegel AK, Schultze-Mosgau S, Wiltfang J. Different substitute biomaterials as potential scaffolds in tissue engineering. Int J Oral Maxillofac Implants. 2006. 21:225–231.4. Springer IN, Terheyden H, Geiss S, Harle F, Hedderich J, Acil Y. Particulated bone grafts: effectiveness of bone cell supply. Clin Oral Implants Res. 2004. 15:205–212.5. Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol. 1996. 1:621–666.
Article6. Jensen SS, Aaboe M, Pinholt EM, Hjorting-Hansen E, Melsen F, Ruyter IE. Tissue reaction and material characteristics of four bone substitutes. Int J Oral Maxillofac Implants. 1996. 11:55–66.7. Fukuta K, Har-Shai Y, Collares MV, Lichten JB, Jackson IT. Comparison of inorganic bovine bone mineral particles with porous hydroxyapatite granules and cranial bone dust in the reconstruction of full-thickness skull defect. J Craniofac Surg. 1992. 3:25–29.
Article8. Haas R, Donath K, Fodinger M, Watzek G. Bovine hydroxyapatite for maxillary sinus grafting: comparative histomorphometric findings in sheep. Clin Oral Implants Res. 1998. 9:107–116.
Article9. Kasabah S, Simunek A, Krug J, Lecaro MC. Maxillary sinus augmentation with deproteinized bovine bone (Bio-Oss) and Impladent dental implant system. Part II. Evaluation of deprotienized bovine bone (Bio-Oss) and implant surface. Acta Medica (Hradec Kralove). 2002. 45:167–171.
Article10. Maiorana C, Redemagni M, Rabagliati M, Salina S. Treatment of maxillary ridge resorption by sinus augmentation with iliac cancellous bone, anorganic bovine bone, and endosseous implants: a clinical and histologic report. Int J Oral Maxillofac Implants. 2000. 15:873–878.11. Merkx MA, Maltha JC, Freihofer HP. Incorporation of composite bone implants in the facial skeleton. Clin Oral Implants Res. 2000. 11:422–429.
Article12. Schwartz Z, Weesner T, van Dijk S, Cochran DL, Mellonig JT, Lohmann CH, et al. Ability of deproteinized cancellous bovine bone to induce new bone formation. J Periodontol. 2000. 71:1258–1269.
Article13. Barboza EP, Duarte ME, Geolas L, Sorensen RG, Riedel GE, Wikesjo UM. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J Periodontol. 2000. 71:488–496.
Article14. Rocchietta I, Dellavia C, Nevins M, Simion M. Bone regenerated via rhPDGF-bB and a deproteinized bovine bone matrix: backscattered electron microscopic element analysis. Int J Periodontics Restorative Dent. 2007. 27:539–545.15. Grzesik WJ, Ivanov B, Robey FA, Southerland J, Yamauchi M. Synthetic integrin-binding peptides promote adhesion and proliferation of human periodontal ligament cells in vitro. J Dent Res. 1998. 77:1606–1612.
Article16. Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000. 1:107–114.
Article17. Mosher DF. Fibronectin. 1989. San Diego: Academic Press.18. Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998. 18:1363–1370.19. Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997. 272:24999–25005.
Article20. Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980. 19:517–525.
Article21. Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996. 88:158–166.
Article22. Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997. 110:861–870.
Article23. Mosher DF, Schad PE, Kleinman HK. Inhibition of blood coagulation factor XIIIa-mediated cross-linking between fibronectin and collagen by polyamines. J Supramol Struct. 1979. 11:227–235.
Article24. Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone. 1999. 25:95–96.
Article25. Okazaki K, Shimizu Y, Xu H, Ooya K. Blood-filled spaces with and without deproteinized bone grafts in guided bone regeneration. A histomorphometric study of the rabbit skull using non-resorbable membrane. Clin Oral Implants Res. 2005. 16:236–243.
Article26. Camargo PM, Wolinsky LE, Ducar JP, Lagos R, Pirih FQ, Jeffcoat M, et al. The effect of fibronectin and a bone xenograft on regenerative treatment: a feasibility study. Compend Contin Educ Dent. 2006. 27:560–568.27. Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Djordjevic M, Kenney EB. The use of bovine porous bone mineral in combination with enamel matrix proteins or with an autologous fibrinogen/fibronectin system in the treatment of intrabony periodontal defects in humans. J Periodontol. 2001. 72:1157–1163.
Article28. Di Bella C, Farlie P, Penington AJ. Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Eng Part A. 2008. 14:483–490.
Article29. Schonmeyr BH, Wong AK, Li S, Gewalli F, Cordiero PG, Mehrara BJ. Treatment of hydroxyapatite scaffolds with fibronectin and fetal calf serum increases osteoblast adhesion and proliferation in vitro. Plast Reconstr Surg. 2008. 121:751–762.
Article30. Lee JA, Ku Y, Park YJ, Koo KT, Kim TI, Seol YJ, et al. The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells. J Korean Acad Periodontol. 2009. 39:45–52.
Article31. Terheyden H, Jepsen S, Moller B, Tucker MM, Rueger DC. Sinus floor augmentation with simultaneous placement of dental implants using a combination of deproteinized bone xenografts and recombinant human osteogenic protein-1. A histometric study in miniature pigs. Clin Oral Implants Res. 1999. 10:510–521.
Article32. Park JB, Lee JY, Park HN, Seol YJ, Park YJ, Rhee SH, et al. Osteopromotion with synthetic oligopeptide-coated bovine bone mineral in vivo. J Periodontol. 2007. 78:157–163.
Article33. Gordjestani M, Dermaut L, De Ridder L, De Waele P, De Leersnijder W, Bosman F. Osteopontin and bone metabolism in healing cranial defects in rabbits. Int J Oral Maxillofac Surg. 2006. 35:1127–1132.
Article34. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986. 205:299–308.
Article35. Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980. 38:176–180.36. Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990. 1:60–68.
Article37. Ascherman JA, Foo R, Nanda D, Parisien M. Reconstruction of cranial bone defects using a quick-setting hydroxyapatite cement and absorbable plates. J Craniofac Surg. 2008. 19:1131–1135.
Article38. Pripatnanont P, Nuntanaranont T, Vongvatcharanon S, Limlertmongkol S. Osteoconductive effects of 3 heat-treated hydroxyapatites in rabbit calvarial defects. J Oral Maxillofac Surg. 2007. 65:2418–2424.
Article39. McAllister BS, Margolin MD, Cogan AG, Buck D, Hollinger JO, Lynch SE. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants. 1999. 14:361–368.40. Klinge B, Alberius P, Isaksson S, Jonsson J. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg. 1992. 50:241–249.
Article41. Schlegel AK. Bio-Oss bone replacement material. Long-term results with Bio-Oss bone replacement material. Schweiz Monatsschr Zahnmed. 1996. 106:141–149.42. Skoglund A, Hising P, Young C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int J Oral Maxillofac Implants. 1997. 12:194–199.43. Yildirim M, Spiekermann H, Handt S, Edelhoff D. Maxillary sinus augmentation with the xenograft Bio-Oss and autogenous intraoral bone for qualitative improvement of the implant site: a histologic and histomorphometric clinical study in humans. Int J Oral Maxillofac Implants. 2001. 16:23–33.44. Pierschbacher MD, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984. 81:5985–5988.
Article45. Rooney MM, Farrell DH, van Hemel BM, de Groot PG, Lord ST. The contribution of the three hypothesized integrin-binding sites in fibrinogen to platelet-mediated clot retraction. Blood. 1998. 92:2374–2381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells
- Comparative Evaluation of Fibrin for Bone Regeneration in Critical Size Calvarial Defects
- Autogenous Calvarial Particulate Bone Grafting in Craniosynostosis
- Correction of craniofacial bony defects using bone dusts and fibrin sealant
- The effect of PRP and fibrin sealant with the deprotenized bovine bone in the rabbit cranium