J Korean Acad Periodontol.
2009 Mar;39(1):45-52. 10.5051/jkape.2009.39.1.45.
The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells
- Affiliations
-
- 1Department of Periodontology, School of Dentistry, Seoul National University, Korea. guy@snu.ac.kr
- 2Craniomaxillofacial Reconstructive Science Major, School of Dentistry, Seoul National University, Korea.
- KMID: 1783516
- DOI: http://doi.org/10.5051/jkape.2009.39.1.45
Abstract
-
PURPOSE: Fibronectin(FN), one of the major components of ECM, mediates wide variety of cellular interactions including cell adhesion, migration, proliferation and differentiation. In this study, we used synthetic peptides based on fibrin binding sites of amino-terminal of FN and evaluated their biologic effects on periodontal ligament(PDL) cells.
MATERIALS AND METHODS
PDL cells were cultured on synthetic oligopeptides coated dishes and examined for cell adhesion, proliferation via confocal microscope. For detection of ERK1/2, cells were plated and Western blot analysis was performed.
RESULTS
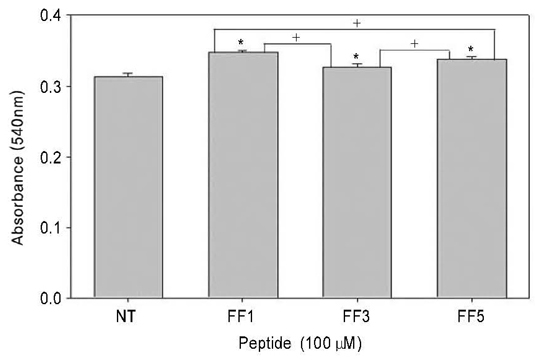
PDL cells on synthetic oligopeptide coated dishes showed enhanced cell adhesion and proliferation. Western blot analysis revealed increased level of ERK1/2 phosphorylation in cells plated on FN fragment containing fibrin-binding domain(FF1 and FF5) coated dishes.
CONCLUSION
These results reveals that FN fragment containing fibrin-binding domain possess an enhanced biologic effect of PDL ligament cells.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects
Ju-A Lee, Young Ku, In-Chul Rhyu, Chong-Pyoung Chung, Yoon-Jeong Park
J Periodontal Implant Sci. 2010;40(5):211-219. doi: 10.5051/jpis.2010.40.5.211.
Reference
-
1. Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and issue remodeling. Bone. 1999; 25:95–96.
Article2. Embery G, Waddington RJ, Hall RC, Last KS. Connective tissue elements as diagnostic aids in periodontology. Periodontol. 2000; 24:193–214.
Article3. Isaka J, Ohazama A, Kobayashi M, et al. Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol. 2001; 72:314–323.
Article4. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article5. Magnus KM, Deane FM. Fibronectin: structure, assembly, and cardiovascular implications. Arterloscler Thromb Vasc Biol. 1998; 18:1363–1370.6. Kapila YL, Lancero H, Johnson PW. The response of periodontal ligament cells to fibronectin. J Periodontol. 1998; 69:1008–1019.
Article7. Lukinmaa PL, Mackie EJ, Thesleff I. Immunohistochemical localization of the matrix glycoproteins-tenascin and ED sequence-containing form of cellular fibronectin-in human permanent teeth and periodontal ligament. J Dent Res. 1991; 70:19–26.
Article8. Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000; 275:21785–21788.
Article9. Agueda AR, Jean ES, Leslie IG. Comparison of the fibrin-binding activities in the N- and C-termini of fibronectin. Biochem J. 1999; 338:375–386.
Article10. Sousa SR, Lamghari M, Sampaio P, Moradas-Ferreira P, Barbosa MA. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J Biomed Mater Res A. 2008; 84:281–290.11. Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997; 110:861–870.
Article12. Grzesik WJ, Ivanov B, Robery FA, Southerland J, Yamauchi M. Synthetic integrin-binding peptides promote adhesion and proliferation of human periodontal ligament cells in vitro. J Dent Res. 1998; 77:1606–1612.
Article13. Kantlehner M, Schaffner P, Finsinger D, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000; 1:107–114.
Article14. De Giglio E, Sabbatini L, Colucci S, Zambonin G. Synthesis, analytical characterization, and osteoblast adhesion properties of RGD-grafted polypyrrole coatings on titanium substrates. J Biomater Sci Polymer Ed. 2000; 10:1073–1083.15. Ku Y, Chung CP, Jang JH. The effect of the surface modification of titanium using a recombinant fragment of fibronectin and vitronectin on cell behavior. Biomaterials. 2005; 26:5153–5157.
Article16. Park JW, Lee SG, Choi BJ, Suh JY. Effects of a cell adhesion molecule coating on the blasted surface of titanium implants on bone healing in the rabbit femur. Int J Oral Maxillofac Implants. 2007; 22:533–541.17. Williams MJ, Phan I, Harvey TS, et al. Solution structure of a pair of fibronectin type 1 modules with fibrin binding activity. J Mol Biol. 1994; 235:1302–1311.
Article18. Blystone SD, Weston LK, Kaplan JE. Fibronectin dependent macrophage fibrin binding. Blood. 1991; 78:2900–2907.
Article19. Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997; 272:24999–25005.
Article20. Grinnel F, Feld M, Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980; 19:517–525.
Article21. Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996; 88:158–166.
Article22. Clark RA, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol. 2004; 122:266–277.
Article23. Knox P, Crooks S, Rimmer CS. Role of fibronectin in the migration of fibroblasts into plasma clots. J Cell Biol. 1986; 102:2318–2323.
Article24. Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996; 84:345–357.
Article25. Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: A link to shape-dependent cell proliferation. Mol Biol Cell. 1995; 6:273–282.
Article26. Leung WK, Wu Q, Hannam PM, McBride BC, Uitto VJ. Treponema denticola may stimulate both epithelial proliferation and apoptosis through MAP kinase signal pathways. J Periodontal Res. 2002; 37:445–455.
Article27. Takeuchi Y, Suzawa M, Kikuchi T, et al. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2 beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997; 11. 14. 272:29309–29316.
Article28. Xiao G, Gopalakrishnan R, Jiang D, et al. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002; 01. 17:101–110.
Article29. Kaori M, Motohiro K, Kenko I, Isao I. Extracellular signaling-regulated kinase1/2 is involved in ascorbic acid-induced osteoblastic differentiation in periodontal ligament cells. J Periodontol. 2007; 78:328–334.30. Kim TI, Jang JH, Lee YM, et al. Design and biological activity of synthetic oligopeptides with Pro-his-Ser-Arg-Asn (PHSRN) and Arg-Gly-Asp(RGD) motifs of human osteoblast-like cell(MG-63) adhesion. Biotechnology letters. 2002; 24:2029–2033.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects
- Effects of nitric oxide on the proliferation and differentiation of human periodontal ligament cells
- The Effect of TGF-beta1 on Cellular Activity of Periodontal Ligament Cells activated by PDGF-BB
- Use biologic fibrin adhesive in otologic surgery: compared with ammonium sulfate fibrin adhesive and tissell®
- Effect of Inorganic Polyphosphate on Cultured Periodontal Ligament Cells