J Korean Med Sci.
2008 Oct;23(5):877-883. 10.3346/jkms.2008.23.5.877.
Protective Effect of Recombinant Adeno-Associated Virus 2/8-Mediated Gene Therapy from the Maternal Hyperphenylalaninemia in Offsprings of a Mouse Model of Phenylketonuria
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Ewha Womans University, Seoul, Korea. jungsc@ewha.ac.kr
- 2Department of Biomedical Sciences, National Institute of Health, Seoul, Korea.
- KMID: 1783076
- DOI: http://doi.org/10.3346/jkms.2008.23.5.877
Abstract
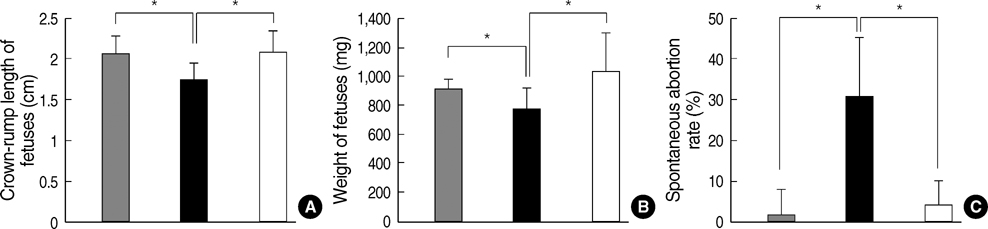
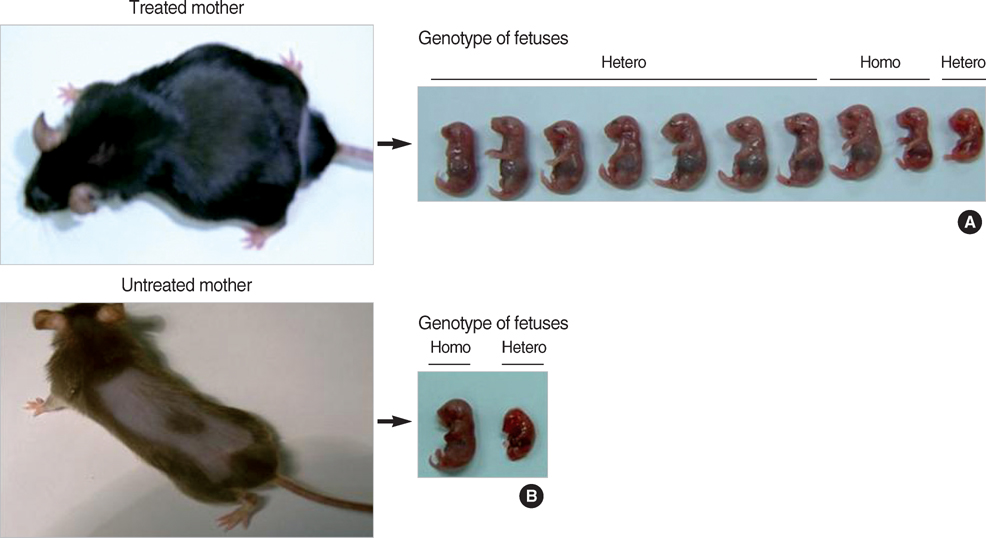
- Phenylketonuria (PKU) is an autosomal recessively inherited metabolic disorder caused by a deficiency of phenylalanine hydroxylase (PAH). The accumulation of phenylalanine leads to severe mental and psychomotor retardation, and the fetus of an uncontrolled pregnant female patient presents with maternal PKU syndrome. We have reported previously on the cognitive outcome of biochemical and phenotypic reversal of PKU in a mouse model, Pah(enu2), by the AAV serotype 2-mediated gene delivery of a human PAH transgene. However, the therapeutic efficacy had been limited to only male PKU mice. In this study, we generated a pseudotyped recombinant AAV2/8-hPAH vector and infused it into female PKU mice through the hepatic portal vein or tail vein. Two weeks after injection, complete fur color change to black was observed in female PKU, as in males. The PAH activity in the liver increased to 65-70% of the wild-type activity in female PKU mice and to 90% in male PKU mice. Plasma phenylalanine concentration in female PKU mice decreased to the normal value. In addition, the offsprings of the treated female PKU mice can rescue from the harmful effect of maternal hyperphenylalaninemia. These results indicate that recombinant AAV2/8-mediated gene therapy is a potential therapeutic strategy for PKU.
MeSH Terms
Figure
Reference
-
1. Lidsky AS, Law ML, Morse HG, Kao FT, Rabin M, Ruddle FH, Woo SL. Regional mapping of the phenylalanine hydroxylase gene and the phenylketonuria locus in the human genome. Proc Natl Acad Sci USA. 1985. 82:6221–6225.
Article2. Hoang L, Byck S, Prevost L, Scriver CR. PAH Mutation Analysis Consortium Database: a database for disease-producing and other allelic variation at the human PAH locus. Nucleic Acids Res. 1996. 24:127–131.3. Erlandsen H, Stevens RC. The structural basis of phenylketonuria. Mol Genet Metab. 1999. 68:103–125.
Article4. Menkes JH. The pathogenesis of mental retardation in phenylketonuria and other inborn errors of amino acid metabolism. Pediatrics. 1967. 39:297–308.5. Pietz J. Neurological aspects of adult phenylketonuria. Curr Opin Neurol. 1998. 11:679–688.
Article6. Koch R, Hanley W, Levy H, Matalon R, Rouse B, Trefz F, Guttler F, Azen C, Friedman E, Platt L, de la Cruz F. Maternal phenylketonuria: an international study. Mol Genet Metab. 2000. 71:233–239.
Article7. Koch R, Azen CG, Friedman EG, Williamson ML. Preliminary report on the effects of diet discontinuation in PKU. J Pediatr. 1982. 100:870–875.
Article8. Cristiano RJ, Smith LC, Woo SL. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci USA. 1993. 90:2122–2126.
Article9. Fang B, Eisensmith RC, Li XH, Finegold MJ, Shedlovsky A, Dove W, Woo SL. Gene therapy for phenylketonuria: phenotypic correction in a genetically deficient mouse model by adenovirus-mediated hepatic gene transfer. Gene Ther. 1994. 1:247–254.10. Nagasaki Y, Matsubara Y, Takano H, Fujii K, Senoo M, Akanuma J, Takahashi K, Kure S, Hara M, Kanegae Y, Saito I, Narisawa K. Reversal of hypopigmentation in phenylketonuria mice by adenovirus-mediated gene transfer. Pediatr Res. 1999. 45:465–473.
Article11. Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, Matsubara Y, Kobayahi E, Okada T, Hoshika A, Ozawa K, Kume A. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004. 11:1081–1086.
Article12. Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004. 56:278–284.
Article13. Ding Z, Georgiev P, Thony B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006. 13:587–593.
Article14. Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, Koeberl DD. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006. 13:457–462.
Article15. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002. 99:11854–11859.
Article16. Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005. 79:214–224.
Article17. Jung SC, Han IP, Limaye A, Xu R, Gelderman MP, Zerfas P, Tirumalai K, Murray GJ, During MJ, Brady RO, Qasba P. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc Natl Acad Sci USA. 2001. 98:2676–2681.
Article18. McDonald JD, Andriolo M, Cali F, Mirisola M, Puglisi-Allegra S, Romano V, Sarkissian CN, Smith CB. The phenylketonuria mouse model: a meeting review. Mol Genet Metab. 2002. 76:256–261.19. Saad AY. Postnatal effects of nicotine on incisor development of albino mouse. J Oral Pathol Med. 1990. 19:426–429.
Article20. Gogu SR, Beckman BS, Agrawal KC. Amelioration of Zidovudine-induced fetal toxicity in pregnant mice. Antimicrob Agents Chemother. 1992. 36:2370–2374.
Article21. Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon Bertoja A, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J, Volk HD. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006. 36:82–94.22. Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003. 102:480–488.
Article23. Berraondo P, Crettaz J, Ochoa L, Paneda A, Prieto J, Troconiz IF, Gonzalez-Aseguinolaza G. Intrahepatic injection of recombinant adeno-associated virus serotype overcomes gender-related differences in liver transduction. Hum Gene Ther. 2006. 17:601–610.24. Chen L, Thung SN, Woo SL. Metabolic basis of sexual dimorphism in PKU mice after genome-targeted PAH gene therapy. Mol Ther. 2007. 15:1079–1085.
Article25. Cho S, McDonald JD. Effect of maternal blood phenylalanine level on mouse maternal phenylketonuria offspring. Mol Genet Metab. 2001. 74:420–425.
Article26. Guttler F, Azen C, Guldberg P, Romstad A, Hanley WB, Levy HL, Matalon R, Rouse BM, Trefz F, de la Cruz F, Koch R. Relationship among genotype, biochemical phenotype and cognitive performance in females with phenylalanine deficiency: report from the Maternal Phenylketonuria Collaborative Study. Pediatrics. 1999. 104:258–262.27. Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, Herzog RW, Nichols TC, Biegel JA, Razavi M, Dake M, Huff D, Flake AW, Couto L, Kay MA, High KA. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001. 4:586–592.
Article28. Jacob M, Muhle C, Park J, Weiss S, Waddington S, Schneider H. No evidence for germ-line transmission following prenatal and early postnatal AAV-mediated gene delivery. J Gene Med. 2005. 7:630–637.29. Schuettrumpf J, Liu JH, Couto LB, Addya K, Leonard DG, Zhen Z, Sommer J, Arruda VR. Inadvertent germline transmission of AAV2 vector: findings in a rabbit model correlate with those in a human clinical trial. Mol Ther. 2006. 13:1064–1073.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant adeno-associated virus mediated gene transfer in a mouse model for homocystinuria
- Mouse models for hepatitis B virus research
- Recombinant AAV Vector with MITF-M Promoter for Melanoma Gene Therapy
- Tissue-specific activation of mitogen-activated protein kinases for expression of transthyretin by phenylalanine and its metabolite, phenylpyruvic acid
- Construction of Recombinant Adeno-Associated Virus Vector (AAVCMVp53) for Human Cervical Cancer Gene Therapy