J Korean Med Sci.
2008 Oct;23(5):852-856. 10.3346/jkms.2008.23.5.852.
Safety and Clinical Responses in Ankylosing Spondylitis after Three Months of Etanercept Therapy
- Affiliations
-
- 1The Hospital for Rheumatic Diseases, Hanyang University, Seoul, Korea. thkim@hanyang.ac.kr
- KMID: 1783072
- DOI: http://doi.org/10.3346/jkms.2008.23.5.852
Abstract
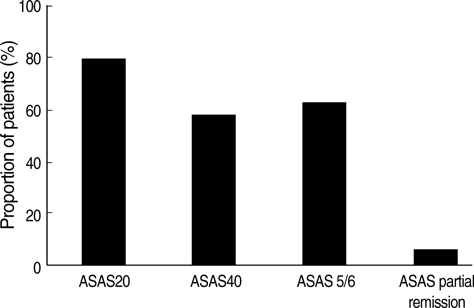
- We aimed to evaluate the safety and clinical responses in Korean ankylosing spondylitis (AS) patients after three months of etanercept therapy. AS patients satisfying the Modified New York Criteria were enrolled. They were assessed for safety and clinical responses at enrollment and after three months of etanercept therapy. A total of 124 patients completed the study. After three months, the rate of ASsessment in AS International Working Group 20% improvement (ASAS 20) response was 79.8%. The rates of ASAS 40 and ASAS 5/6 responses were 58.5 and 62.8%, respectively. Significant improvement of Korean version of Bath AS Disease Activity Index (KBASDAI) (p<0.0001), Bath AS Functional Activity Index (BASFI) (p<0.0001), and Bath AS Metrology Index (BASMI) (p=0.0009) were achieved after three months. Quality of life was also significantly improved after three months, as demonstrated by scores for SF-36 (p<0.0001) and EQ-5D (p<0.0001). Erythrocyte sedimentation rate and C-reactive protein were significantly decreased (p<0.0001, p<0.0001, respectively). None of the patients developed tuberculosis and there were no serious adverse event. AS patients with inadequate response to conventional therapy showed significant clinical improvement without serious adverse events after three months of etanercept therapy.
MeSH Terms
Figure
Cited by 3 articles
-
Etanercept Treatment in Ankylosing Spondylitis Hip Lesions
Young-Chang Kim, Sang Won Moon
Hip Pelvis. 2013;25(2):135-140. doi: 10.5371/hp.2013.25.2.135.A Case of Ankylosing Spondylitis with Cricoarytenoid Arthritis
Ju Kyeon Yim, Sung Dong Kwak, Jae Young Park, Jae Hong Cheon, Sung Yeol Choi, Choong Won Lee
J Korean Rheum Assoc. 2009;16(2):161-166. doi: 10.4078/jkra.2009.16.2.161.The Epidemiology and Treatment of Ankylosing Spondylitis in Korea
Seong-Ryul Kwon, Tae-Hwan Kim, Tae-Jong Kim, Won Park, Seung Cheol Shim
J Rheum Dis. 2022;29(4):193-199. doi: 10.4078/jrd.22.0023.
Reference
-
1. Ahearn JM, Hochberg MC. Epidemiology and genetics of ankylosing spondylitis. J Rheumatol Suppl. 1988. 16:22–28.2. Lee JH, Jun JB, Jung S, Bae SC, Yoo DH, Kim TY, Kim SY, Kim TH. Higher prevalence of peripheral arthritis among ankylosing spondylitis patients. J Korean Med Sci. 2002. 17:669–673.
Article3. Boonen A, van der Heijde D, Landewe R, Guillemin F, Rutten-van Molken M, Dougados M, Mielants H, de Vlam K, van der Tempel H, Boesen S, Spoorenberg A, Schouten H, van der Linden S. Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis. 2003. 62:732–740.4. Sturrock RD, Hart FD. Double-blind cross-over comparison of indomethacin, flurbiprofen, and placebo in ankylosing spondylitis. Ann Rheum Dis. 1974. 33:129–131.
Article5. Chen J, Liu C. Is sulfasalazine effective in ankylosing spondylitis? A systematic review of randomized controlled trials. J Rheumatol. 2006. 33:722–731.6. Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2004. CD002822.
Article7. Crew MD, Effros RB, Walford RL, Zeller E, Cheroutre H, Brahn E. Transgenic mice expressing a truncated Peromyscus leucopus TNF-alpha gene manifest an arthritis resembling ankylosing spondylitis. J Interferon Cytokine Res. 1998. 18:219–225.8. Gratacos J, Collado A, Filella X, Sanmarti R, Canete J, Llena J, Molina R, Ballesta A, Munoz-Gomez J. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994. 33:927–931.9. Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, Eggens U, Distler A, Sieper J. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995. 38:499–505.
Article10. Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002. 346:1349–1356.11. Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, Mola EM, Salvarani C, Sanmarti R, Sany J, Sibilia J, Sieper J, van der Linden S, Veys E, Appel AM, Fatenejad S. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004. 63:1594–1600.
Article12. van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, Braun J. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005. 52:582–591.
Article13. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984. 27:361–368.14. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994. 21:2286–2291.15. Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994. 21:2281–2285.16. Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994. 21:1694–1698.17. Kim MH, Cho YS, Uhm WS, Kim S, Bae SC. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res. 2005. 14:1401–1406.
Article18. Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001. 44:1876–1886.
Article19. Brandt J, Listing J, Sieper J, Rudwaleit M, van der Heijde D, Braun J. Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis. 2004. 63:1438–1444.20. Guideline for the treatment of latent tuberculosis in patients receiving biologic agents. Korea Food and Drug Administration. 2004. Available online at http://www.kfda.go.kr.21. Yun JH, Kang JM, Kim KS, Kim SH, Kim TW, Park YW, Sung YK, Sohn JH, Song BJ, Uhm WS, Yoon HJ, Lee OY, Lee JH, Lee CB, Lee CH, Jung WT, Choe JY, Choi HS, Han DS, Bae SC. Health-related quality of life in Korean patients with chronic diseases. J Korean Rheum Assoc. 2004. 11:263–274.22. Seong SS, Choi CB, Sung YK, Park YW, Lee HS, Uhm WS, Kim TH, Jun JB, Yoo DH, Lee OY, Bae SC. Health-related quality of life using EQ-5D in Koreans. J Korean Rheum Assoc. 2004. 11:254–262.23. Brandt J, Listing J, Haibel H, Sorensen H, Schwebig A, Rudwaleit M, Sieper J, Braun J. Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford). 2005. 44:342–348.
Article24. Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Golder W, Gromnica-Ihle E, Kellner H, Schneider M, Sorensen H, Zeidler H, Reddig J, Sieper J. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum. 2003. 48:2224–2233.
Article25. Baraliakos X, Brandt J, Listing J, Haibel H, Sorensen H, Rudwaleit M, Sieper J, Braun J. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: clinical and magnetic resonance imaging data. Arthritis Rheum. 2005. 53:856–863.
Article26. Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, Sieper J. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998. 41:58–67.
Article27. Davis JC Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, Kivitz A, Fleischmann R, Inman R, Tsuji W. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003. 48:3230–3236.
Article28. Jauregui E, Conner-Spady B, Russell AS, Maksymowych WP. Clinimetric evaluation of the bath ankylosing spondylitis metrology index in a controlled trial of pamidronate therapy. J Rheumatol. 2004. 31:2422–2428.29. Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, Sorensen H, Zeidler H, Thriene W, Sieper J. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002. 359:1187–1193.
Article30. Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis. 2004. 63:665–670.31. Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003. 48:2122–2127.32. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, Carreno L, Figueroa M. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005. 52:1766–1772.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TNF Inhibitors and Uveitis in Ankylosing Spondylitis
- Etanercept Treatment in Ankylosing Spondylitis Hip Lesions
- Anti-TNF-alpha Therapy for Ankylosing Spondylitis
- A Case of Ankylosing Spondylitis Accompanying Sarcoidosis
- A Case of Varicelliform Zoster in a Patient Treated with Etanercept for Ankylosing Spondylitis