Evaluation of Automated Architect Syphilis TP as a Diagnostic Laboratory Screening Test for Syphilis
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University Hospital, Seoul, Korea. eqcho1ku@korea.ac.kr
- KMID: 1781583
- DOI: http://doi.org/10.3343/kjlm.2008.28.6.475
Abstract
- BACKGROUND
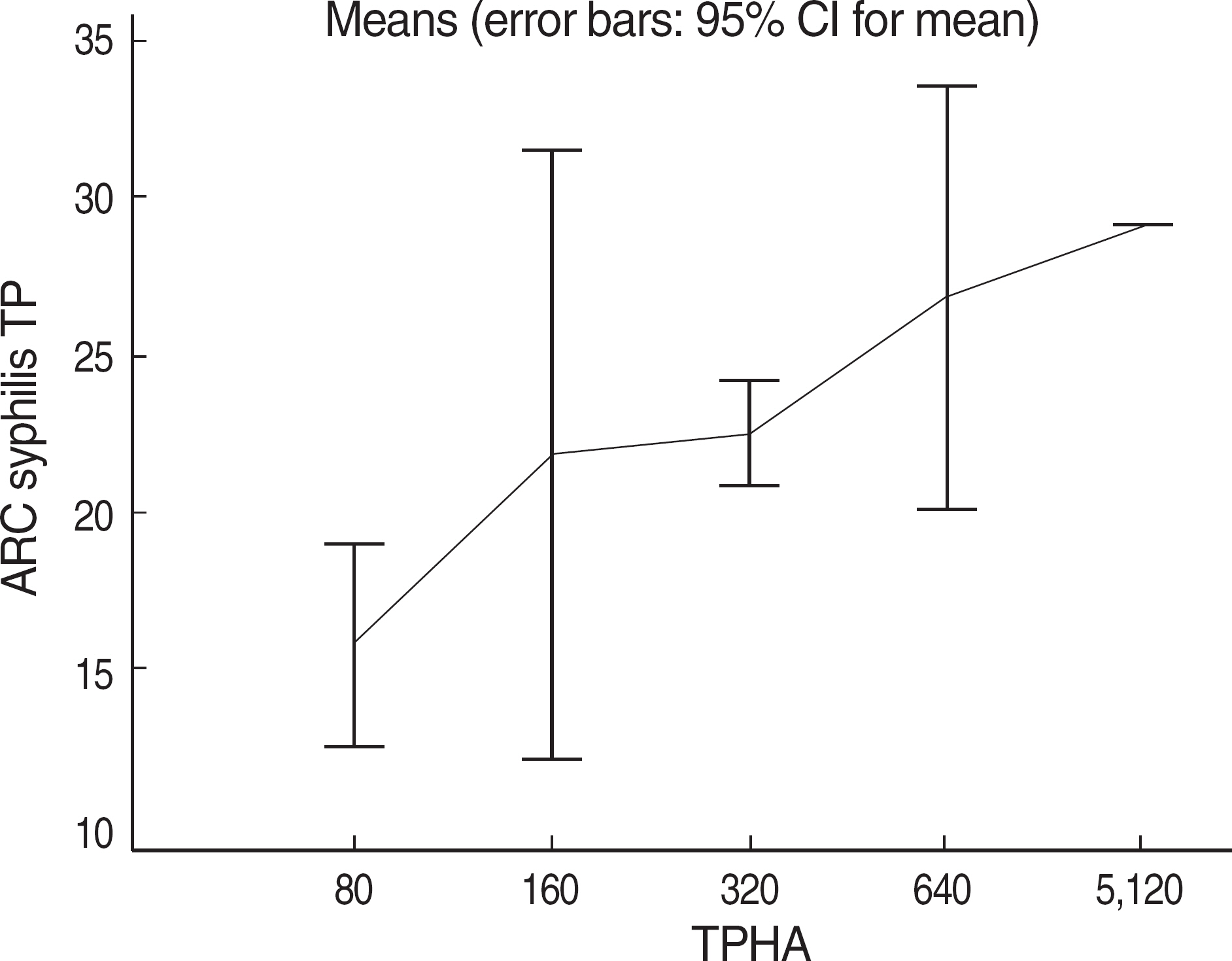
The aim of the study was to establish a new syphilis test algorithm using Architect Syphilis TP (Abbott Japan, Japan: AST), a fully automated treponemal antibody test, as a screening test in a university hospital laboratory. We evaluated performance characteristics of AST in various patient groups. METHODS: A total of 1,357 serum samples obtained from patients at a university hospital from June to August, 2008 were categorized into checkup, preoperative, other diseases, diagnosis (clinically suspected of syphilis), and follow up groups. We compared the results of AST with those of RPR (N=1,276) or Treponema pallidum hemagglutination assay (TPHA, N=81). Samples with discrepant results between RPR or TPHA and AST were retested by fluorescent treponemal antibody absorption test (FTA-ABS) and all patients' clinical records were thoroughly reviewed. RESULTS: The positive rate of AST was significantly higher than that of RPR in preoperative and other diseases groups and was the same as that of RPR in diagnosis group. There were no significant differences in check up and follow up groups. The results of AST showed 97.4% (1,243/1,276) and 97.5% (79/81) concordance rates with those of RPR and TPHA, respectively. Among 26 RPR-AST discrepant and FTA-ABS confirmed cases, there were 20 RPR false-negatives, 4 RPR false-positives, 1 AST false-negative, and 1 AST false-positive. CONCLUSIONS: Based on the results and literature review, we established a new syphilis test algorithm using AST as a screening test, which would be helpful for detection of more syphilis patients including latent infections.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Algorithms
Autoanalysis
Child
Child, Preschool
False Positive Reactions
Female
Fluorescent Treponemal Antibody-Absorption Test/methods
Hemagglutination Tests/methods
Humans
Male
Middle Aged
Reagent Kits, Diagnostic
Sensitivity and Specificity
Syphilis/*diagnosis
Syphilis Serodiagnosis/*methods
Figure
Cited by 4 articles
-
Secondary Syphilis with Nodular Vasculitis Mimicking Behçet's Disease
Jaemin Jo, Sang Taek Heo, Jae Wang Kim, Jinseok Kim, Jung Re Yu
Infect Chemother. 2013;45(4):451-454. doi: 10.3947/ic.2013.45.4.451.Recent Trends in Clinical Observation of Syphilis and Consideration for Laboratory Tests
Kyu Chul Choi, Ji Young Song
J Korean Med Assoc. 2009;52(11):1100-1106. doi: 10.5124/jkma.2009.52.11.1100.Establishment and Multicenter Evaluation of a National Reference Panel for Syphilis Antibodies in Korea
Hee Jin Huh, Seok Lae Chae, Deok-Ja Oh, Quehn Park, Chae Seung Lim, Tae Hyun Um, Yun Mi Park, Young Joo Cha
Lab Med Online. 2014;4(1):36-42. doi: 10.3343/lmo.2014.4.1.36.Comparison of Quantitative Results among Two Automated Rapid Plasma Reagin (RPR) Assays and a Manual RPR Test
Yeong Sic Kim, Jehoon Lee, Hae Kyung Lee, Hyunjung Kim, Hi Jeong Kwon, Ki Ouk Min, Eun Joo Seo, Soo-Young Kim
Korean J Lab Med. 2009;29(4):331-337. doi: 10.3343/kjlm.2009.29.4.331.
Reference
-
1.Philip SS., Jacobs RA. Spirochetal Infections. McPhee SJ., Papadakis MA, et al. Current medical diagnosis & treatment. 2009. http://www.accessmedicine.com/content.aspx?aID=18592. (last opened on Nov 2008).2.Korea Centers for Disease Control and Prevention. Disease web statistics system. http://stat.cdc.go.kr/Index_List.aspx. (updated on Oct 2008).3.Jang HC., Cho JH., Park WB., Lee KD., Lee CS., Kim HB, et al. Outbreak of primary and secondary syphilis among HIV sero-positive patients in Korea. Infection and Chemotherapy. 2004. 36:366–72. (장희창, 조재현, 박완범, 이기덕, 이창섭, 김홍빈 등. 인간 면역부전 바이러스(HIV) 감염자 사이에서의 1기 및 2기 매독의 유행. 감염과 화학요법 2004;36: 366-72.).4.Lee SH., Suh DH., Cho KH., Eun HC. Two cases of unusual manifestations of secondary syphilis accompanied by human immunodeficiency virus infection. Korean J Dermatol. 2003. 41:354–9. (이승호, 서대헌, 조광현, 은희철. Human immunodeficiency virus 감염에 동반된 비전형적인 피부소견을 보인 2기 매독 2예. 대한피부과학회지 2003;41: 354-9.).5.Kang SK., Kim ES., Lee MW., Choi JH., Sung KJ., Moon KC, et al. Two cases of secondary syphilis accompanied by acquired immunodeficiency syndrome. Korean J Dermatol. 2002. 40:428–32. (강숙경, 김은성, 이미우, 최지호, 성경제, 문기찬등. 후천성면역결핍증에동반된 2기매독 2예. 대한피부과학회지 2002;40: 428-32.).6.Song EY., Yang JS., Chae SL., Kim S., Choi YS., Cha YJ. Current status of external quality assessment of syphilis test in Korea. Korean J Lab Med. 2008. 28:207–13. (송은영, 양주석, 채석래, 김세림, 최영숙, 차영주. 국내 매독검사의 외부신빙도조사 현황. 대한진단검사의학회지 2008;28: 207-13.).
Article7.Pope V., Ari MD., Schriefer ME., Levett PN. Immunologic methods for diagnosis of spirochetal disease. Detrick B, Hamilton RG, editors. Manual of molecular and clinical laboratory immunology. 7th ed.Washington DC: ASM Press;2006. p. 477–92.8.Goh BT., van Voorst Vader PC. European guideline for the management of syphilis. Int J STD AIDS. 2001. 12(S):S14–26.
Article9.Kim YH., Kim HR., Park C., Seo MY., Park J. Comparative sensitivity of Architect syphilis TP with other antibody tests. J Lab Med Qual Assur. 2008. 30:217–21. (김영휴, 김형락, 박철, 서민영, 박준석. Architect Syphilis TP를 이용한 매독감염항체 검사 민감도의 비교평가. 임상검사와정도관리 2008;30: 217-21.).10.Yoshioka N., Deguchi M., Kagita M., Kita M., Watanabe M., Asari S, et al. Evaluation of a chemiluminescent microparticle immunoassay for determination of Treponema pallidum antibodies. Clin Lab. 2007. 53:597–603.11.Egglestone SI., Turner AJ. Serological diagnosis of syphilis. PHLS syphilis serology working group. Commun Dis Public Health. 2000. 3:158–62.12.Young H. Guidelines for serological testing for syphilis. Sex Transm Infect. 2000. 76:403–5.
Article13.U.S. Food and Drug Administration. Cleared tests for Treponema pallidum (Syphilis). http://www.fda.gov/CBER/tissue/prod.htm#syp. (Updated on Aug 2008).14.Centers for Disease Control and Prevention (CDC). Syphilis testing algorithms using treponemal tests for initial screening–four laboratories, New York city, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008. 57:872–5.15.Huh HJ., Lee KK., Kim ES., Chae SL. Analysis of positive results in Mediace rapid plasma reagin and Treponema pallidum latex agglutination as the automated syphilis test. Korean J Lab Med. 2007. 27:324–9. (허희진, 이교관, 김의석, 채석래. 자동화 매독검사인 Mediace Rapid Plasma Reagin과 Treponema pallidum Latex Agglutination 양성결과분석. 대한진단검사의학회지 2007;27: 324-9.).16.Centers for disease control and prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006. 55:1–94.17.Hagedorn HJ., Kraminer-Hagedorn A., De Bosschere K., Hulstaert F., Pottel H., Zrein M. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J Clin Microbiol. 2002. 40:973–8.
Article18.Backhouse JL., Nesteroff SI. Treponema pallidum western blot: comparison with the FTA-ABS test as a confirmatory test for syphilis. Diagn Microbiol Infect Dis. 2001. 39:9–14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Sensitivity of ARCHITECT Syphilis TP with Other Antibody Tests
- Comparative Performance of the Reverse Algorithm Using Architect Syphilis TP Versus the Traditional Algorithm Using Rapid Plasma Reagin in Florida's Public Health Testing Population
- Recent Trends in Clinical Observation of Syphilis and Consideration for Laboratory Tests
- Comparison of Auto RPR Plus and Auto TPIM Plus with Mediace RPR and Abbott Syphilis TP for Serologic Diagnosis of Syphilis
- Acquired Secondary Syphilis in Early Childhood