Korean J Lab Med.
2007 Oct;27(5):330-337. 10.3343/kjlm.2007.27.5.330.
Evaluation of Viva-E Drug Testing System
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. suddenbz@skku.edu
- KMID: 1781531
- DOI: http://doi.org/10.3343/kjlm.2007.27.5.330
Abstract
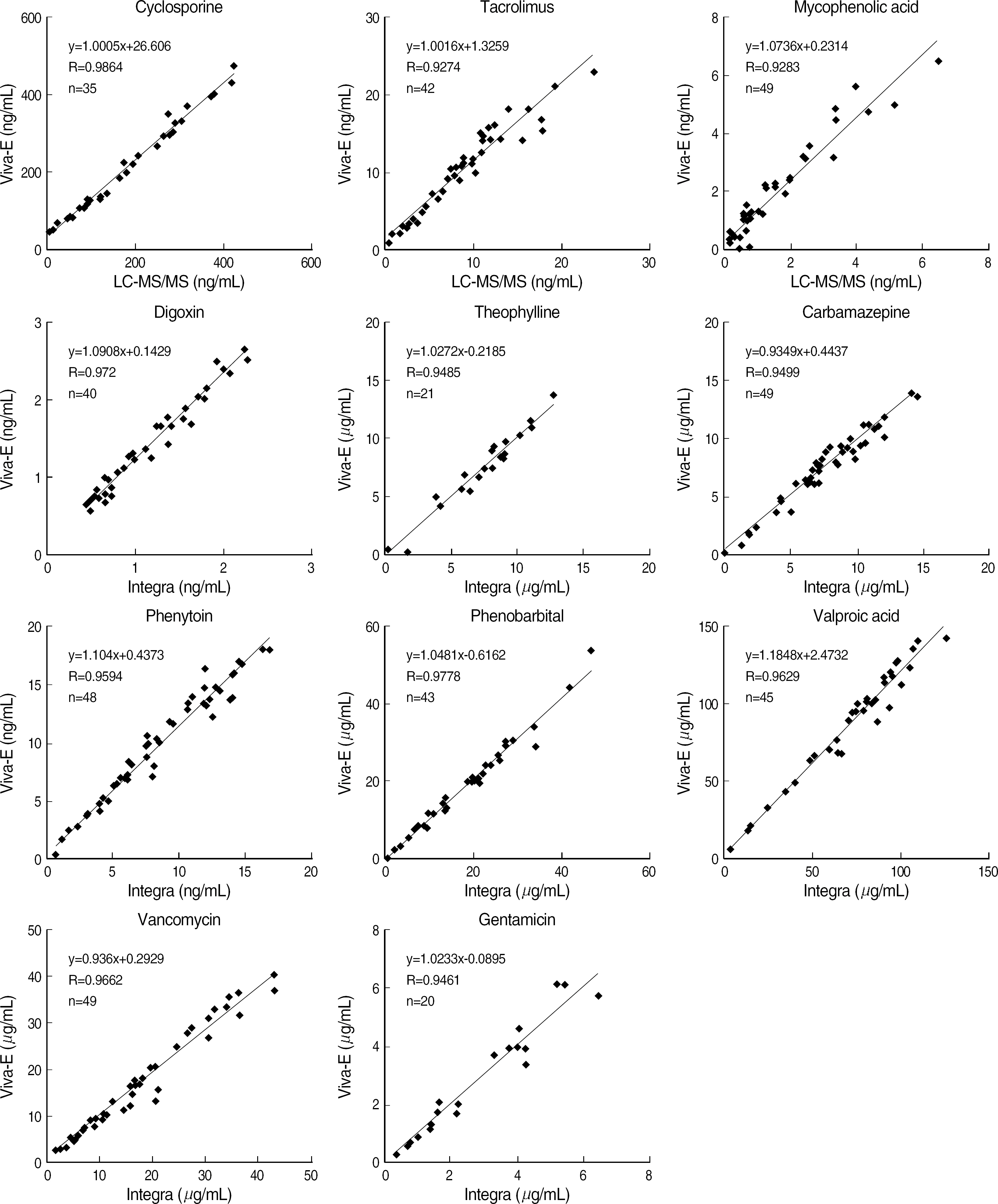
- BACKGROUND: The importance and usefulness of therapeutic drug monitoring (TDM) have been emphasized, and analysis of drugs has been increased in clinical laboratories. We evaluated the analytical performance and clinical usefulness of a recently introduced enzyme multiplied immunoassay instrument, Viva-E Drug Testing System (Dade Behring Inc., USA). METHODS: Using patients' samples and quality control material, we evaluated the analytical performance of Viva-E for a total of 11 drugs (cyclosporine, tacrolimus, mycophenolic acid, valproic acid, digoxin, theophylline, carbamazepine, phenytoin, phenobarbital, vancomycin, and gentamicin) with respect to linearity, precision, and correlations with other methods according to CLSI guidelines. Cobas Integra 800 (Roche Diagnostics, Switzerland) and API 4000 LC-MS/MS System (Applied Biosystems, USA) were used to make a comparison. In addition, we analyzed analysis time. RESULTS: Viva-E showed a good linearity (r2 > or = 0.97) for all items. Within-run CVs were within 5% and total CVs were within 10% for all drugs except for tacrolimus and digoxin at low concentrations. The system correlated well with the other methods (r=0.9283-0.9778). The time required for reporting the first sample was 11 min and the analysis time was 1.1 min. CONCLUSIONS: Since Viva-E showed a good analytical performance required for TDM in its linearity, precision, and accuracy with its wide drug menus including cyclosporine, tacrolimus, and mycophenolic acid, stat and random accessing functions, and the consolidation to a single workstation, it could be very useful in the clinical laboratory for various needs.
MeSH Terms
Figure
Cited by 1 articles
-
Performance Evaluation of the Syva EMIT Methotrexate Assay on the Toshiba 200FR NEO
Jinsook Lim, Jimyung Kim, Yong-Hak Sohn, Sun Hoe Koo, Gye Cheol Kwon
Lab Med Online. 2014;4(4):187-190. doi: 10.3343/lmo.2014.4.4.187.
Reference
-
1.Kwon HJ., Lee EH. Therapeutic drug monitoring, heavy metals, toxicology. The Korean Society for Laboratory Medicine. Laboratory medicine. 3rd ed.Seoul: Korea medical book publisher Co.;2001. p. 169–73. (권희정및이은희. 치료적약물농도모니터링, 중금속, 독물검사. 대한진단검사의학회 편. 진단검사의학. 3판. 고려의학 2001 169-173.).2.Kim JH. 30 Years of the Korean association of quality assurance for clinical laboratory-TDM. J Lab Med Qual Assur. 2006. 28(S2):S425–33. (김정호. 임상검사정도관리 30년-TDM 분과. 임상검사와 정도관리2006;28(부록2): S425-33.).3.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; approved guideline. Document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.4.Clinical and Laboratory Standards Institute. Evaluation of the precision performance of clinical chemistry devices; approved guideline. Document EP05-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.5.Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline. Document EP09-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.6.Wu AH. A selected history and future of immunoassay development and applications in clinical chemistry. Clin Chim Acta. 2006. 369:119–24.
Article7.Massey HD., McPherason RA. Immunology and immunopathology. McPherson RA, Pincus MR, editors. Clinical diagnosis and management by laboratory methods. 21th ed.Philadelphia: Saunders Elsevier;2007. p. 793–818.8.Lee SY. Evaluation of methods for TDM. Korean J Lab Med. 2007. 27(S1):S200–5. (이수연. TDM 검사법및장비의선택. 대한진단검사의학회지 2007;27(부록1): S200-5.).9.Armstrong VW., Oellerich M. Critical evaluation of methods for therapeutic drug monitoring. Evans WE, editor. Applied pharmacokinetics and pharmacodynamics. 4th ed.Baltimore: Lippincott Williams & Wilkins;2006. p. 30–9.10.Boer K., Deufel T., Schmidt D., Streck S., Kiehntopf M. Application of the EMIT 2000 tacrolimus assay on the Abbott Architect c8000 high volume clinical chemistry analyzer. Clin Biochem. 2006. 39:1041–3.
Article11.LeGatt DF., Shalapay CE., Cheng SB. The EMIT 2000 tacrolimus assay: an application protocol for the Beckman Synchron LX20 PRO analyzer. Clin Biochem. 2004. 37:1022–30.
Article12.Akbas SH., Yavuz A., Tuncer M., Yurdakonar E., Akcit F., Gurkan A, et al. Evaluation of the new EMIT tacrolimus assay in kidney and liver transplant recipients. Transplant Proc. 2004. 36:86–8.
Article13.Napoli KL. Is microparticle enzyme-linked immunoassay (MEIA) reliable for use in tacrolimus TDM? Comparison of MEIA to liquid chromatography with mass spectrometric detection using longitudinal trough samples from transplant recipients. Ther Drug Monit. 2006. 28:491–504.
Article14.EMIT 2000 Tacrolimus Application sheet (package insert). Dade Behring Inc;2004.15.Saccoia NC., Hackett LP., Morris RG., Ilett KK. Enzyme-multiplied immunoassay (EMIT 2000) digoxin assay compared with fluorescence polarization immunoassay and Amerlex 125I-radioimmunoassay at two Australian centers. Ther Drug Monit. 1996. 18:672–7.16.Alain J., Forest JC. Adaptation of the Syva EMIT-cad digoxin enzyme immunoassay technique on the Instrumentation Laboratory Multistat-III microcentrifugal analyzer. Ther Drug Monit. 1984. 6:489–92.17.Kim JP., Kim M., Yun M., Lee CJ., Shin JH., Suh SP, et al. Evaluation of Vitros 950 for quantitative analysis of digoxin and theophylline. Korean J Clin Pathol. 1999. 19:409–13. (김종필, 김민, 윤명, 이창재, 신종희, 서순팔 등. Vitros 950을 이용한 Digoxin 및 Theophylline 측정에대한평가. 대한임상병리학회지 1999;19: 409-13.).18.Jiang F., Wilhite TR., Smith CH., Landt M. A new digoxin immunoassay substantially free of interference by digoxin immunoreactive factor. Ther Drug Monit. 1995. 17:184–8.
Article19.Ferreri LF., Raisys VA., Opheim KE. Analysis of digoxin concentrations in serum by fluorescence polarization immunoassay: an evaluation. J Anal Toxicol. 1984. 8:138–40.
Article20.EMIT 2000 Digoxin Application sheet (package insert). Dade Behring Inc;2004.21.Wong SH. Therapeutic drug monitoring for immunosuppressants. Clin Chim Acta. 2001. 313:241–53.
Article22.Holt DW., Johnston A., Roberts NB., Tredger JM., Trull AK. Methodological and clinical aspects of cyclosporin monitoring: report of the Association of Clinical Biochemists task force. Ann Clin Biochem. 1994. 31:420–46.
Article23.Schutz E., Svinarov D., Shipkova M., Niedmann PD., Armstrong VW., Wieland E, et al. Cyclosporin whole blood immunoassays (AxSYM, CEDIA, and Emit): a critical overview of performance characteristics and comparison with HPLC. Clin Chem. 1998. 44:2158–64.24.Steimer W. Performance and specificity of monoclonal immunoassays for cyclosporine monitoring: how specific is specific? Clin Chem. 1999. 45:371–81.
Article25.Hamwi A., Veitl M., Manner G., Ruzicka K., Schweiger C., Szekeres T. Evaluation of four automated methods for determination of whole blood cyclosporine concentrations. Am J Clin Pathol. 1999. 112:358–65.
Article26.Terrell AR., Daly TM., Hock KG., Kilgore DC., Wei TQ., Hernandez S, et al. Evaluation of a no-pretreatment cyclosporin A assay on the Dade Behring dimension RxL clinical chemistry analyzer. Clin Chem. 2002. 48:1059–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Syva EMIT Methotrexate Assay on the Toshiba 200FR NEO
- Introduction to an Open Source Internet-Based Testing Program for Medical Student Examinations
- Evaluation of Mycobacteria Growth Indicator Tube for Drug Susceptibility Testing of Mycobacterium tuberculosis Using MGIT 960 System
- Update on the diagnosis of drug allergy
- Multi-center Performance Evaluations of Tacrolimus and Cyclosporine Electrochemiluminescence Immunoassays in the Asia-Pacific Region