Ann Lab Med.
2013 Jan;33(1):60-64. 10.3343/alm.2013.33.1.60.
Identification of Two Novel NPM1 Mutations in Patients with Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea. MWSeong@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, National Medical Center, Seoul, Korea.
- KMID: 1781299
- DOI: http://doi.org/10.3343/alm.2013.33.1.60
Abstract
- BACKGROUND
Genetic abnormalities in adult AML are caused most frequently by somatic mutations in exon 12 of the NPM1 gene, which is observed in approximately 35% of AML patients and up to 60% of patients with cytogenetically normal AML (CN-AML).
METHODS
We performed mutational analysis, including fragment analysis and direct sequencing of exon 12 of the NPM1 gene, on 83 AML patients to characterize the NPM1 mutations completely.
RESULTS
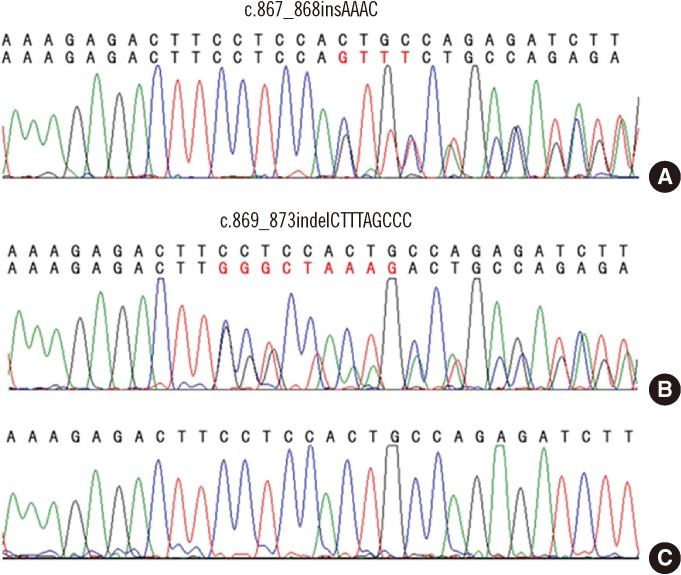
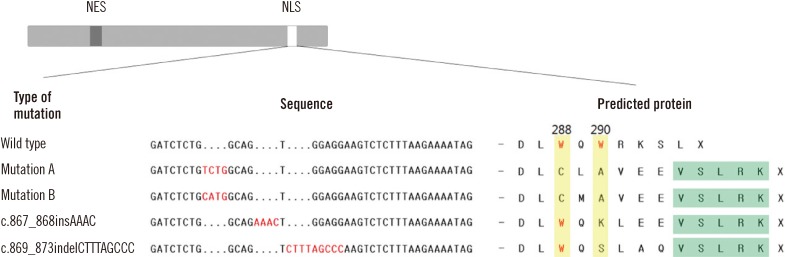
In this study, NPM1 mutations were identified in 19 (22.9%) of the 83 AML patients and in 12 (42.9%) of the 28 CN-AML patients. Among the 19 patients with NPM1 mutations, type A NPM1 mutations were identified in 16 (84.2%) patients, whereas non-A type NPM1 mutations were observed in 3 (15.8%) patients. Two of the 3 non-A type NPM1 mutations were novel: c.867_868insAAAC and c.869_873indelCTTTAGCCC. These 2 novel mutant proteins display a nuclear export signal motif (L-xxx-L-xx-V-x-L) less frequently and exhibit a mutation at tryptophan 290 that disrupts the nucleolar localization signal.
CONCLUSIONS
This study suggests that novel NPM1 mutations may be non-rare and that supplementary sequence analysis is needed along with conventional targeted mutational analysis to detect non-A types of NPM1 mutations.
Keyword
MeSH Terms
Figure
Reference
-
1. Eirín-López JM, Frehlick LJ, Ausió J. Long-term evolution and functional diversification in the members of the nucleophosmin/nucleoplasmin family of nuclear chaperones. Genetics. 2006; 173:1835–1850. PMID: 16751661.2. Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006; 6:493–505. PMID: 16794633.
Article3. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005; 352:254–266. PMID: 15659725.
Article4. Rau R, Brown P. Nucleophosmin (NPM1) mutations in adult and childhood acute myeloid leukaemia: towards definition of a new leukaemia entity. Hematol Oncol. 2009; 27:171–181. PMID: 19569254.
Article5. Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005; 106:3740–3746. PMID: 16051734.6. Yu Y, Maggi LB Jr, Brady SN, Apicelli AJ, Dai MS, Lu H, et al. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol Cell Biol. 2006; 26:3798–3809. PMID: 16648475.
Article7. Nishimura Y, Ohkubo T, Furuichi Y, Umekawa H. Tryptophans 286 and 288 in the C-terminal region of protein B23.1 are important for its nucleolar localization. Biosci Biotechnol Biochem. 2002; 66:2239–2242. PMID: 12450141.
Article8. Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007; 109:874–885. PMID: 17008539.
Article9. Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood. 2006; 107:4514–4523. PMID: 16455950.
Article10. Mariano AR, Colombo E, Luzi L, Martinelli P, Volorio S, Bernard L, et al. Cytoplasmic localization of NPM in myeloid leukemias is dictated by gain-of-function mutations that create a functional nuclear export signal. Oncogene. 2006; 25:4376–4380. PMID: 16501600.
Article11. Koh Y, Park J, Bae EK, Ahn KS, Kim I, Bang SM, et al. Non-A type nucleophosmin 1 gene mutation predicts poor clinical outcome in de novo adult acute myeloid leukemia: differential clinical importance of NPM1 mutation according to subtype. Int J Hematol. 2009; 90:1–5. PMID: 19484332.
Article12. Park BG, Chi HS, Park SJ, Min SK, Jang S, Park CJ, et al. Clinical implications of non-A-type NPM1 and FLT3 mutations in patients with normal karyotype acute myeloid leukemia. Acta Haematol. 2012; 127:63–71. PMID: 22104247.
Article13. Kim YK, Kim HN, Lee SR, Ahn JS, Yang DH, Lee JJ, et al. Prognostic significance of nucleophosmin mutations and FLT3 internal tandem duplication in adult patients with cytogenetically normal acute myeloid leukemia. Korean J Hematol. 2010; 45:36–45. PMID: 21120161.
Article14. Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood. 2011; 117:1109–1120. PMID: 21030560.
Article15. Falini B, Bolli N, Liso A, Martelli MP, Mannucci R, Pileri S, et al. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: molecular basis and clinical implications. Leukemia. 2009; 23:1731–1743. PMID: 19516275.
Article16. Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005; 106:3747–3754. PMID: 16109776.
Article17. Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006; 107:4011–4020. PMID: 16455956.
Article18. Ahmad F, Mandava S, Das BR. Mutations of NPM1 gene in de novo acute myeloid leukaemia: determination of incidence, distribution pattern and identification of two novel mutations in Indian population. Hematol Oncol. 2009; 27:90–97. PMID: 19365794.19. Yan L, Chen S, Liang J, Feng Y, Cen J, He J, et al. Analysis of NPM1 gene mutations in Chinese adults with acute myeloid leukemia. Int J Hematol. 2007; 86:143–146. PMID: 17875528.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reclassification of Acute Myeloid Leukemia According to the 2016 WHO Classification
- Effects of Somatic Mutations Are Associated with SNP in the Progression of Individual Acute Myeloid Leukemia Patient: The Two-Hit Theory Explains Inherited Predisposition to Pathogenesis
- Molecular Risk Stratification using Next-generation Sequencing in Acute Myeloid Leukemia
- A Pediatric Case of Acute Myeloid Leukemia with t(3;5)(q25;q34)
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia