Yonsei Med J.
2012 Jan;53(1):172-180. 10.3349/ymj.2012.53.1.172.
Gene Expression in Rat Hearts Following Oral Administration of a Single Hepatotoxic Dose of Acetaminophen
- Affiliations
-
- 1Division of Cardiology, Department of Pediatrics, College of Medicine, Eulji University, Daejeon, Korea. sunmijin@medimail.co.kr
- 2Department of Pediatrics, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3College of Pharmacy, Dongduk Women's University, Seoul, Korea.
- 4Department of Pediatrics, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 1779703
- DOI: http://doi.org/10.3349/ymj.2012.53.1.172
Abstract
- PURPOSE
Toxicity caused by acetaminophen and its toxic mechanisms in the liver have been widely studied, including effects involving metabolism and oxidative stress. However, its adverse effects on heart have not been sufficiently investigated. This study evaluated the cardiac influence and molecular events occurring within the myocardium in rats treated with a dose of acetaminophen large enough to induce conventional liver damage.
MATERIALS AND METHODS
Male rats were orally administered a single dose of acetaminophen at 1,000 mg/kg-body weight, and subsequently examined for conventional toxicological parameters and for gene expression alterations to both the heart and liver 24 hours after administration.
RESULTS
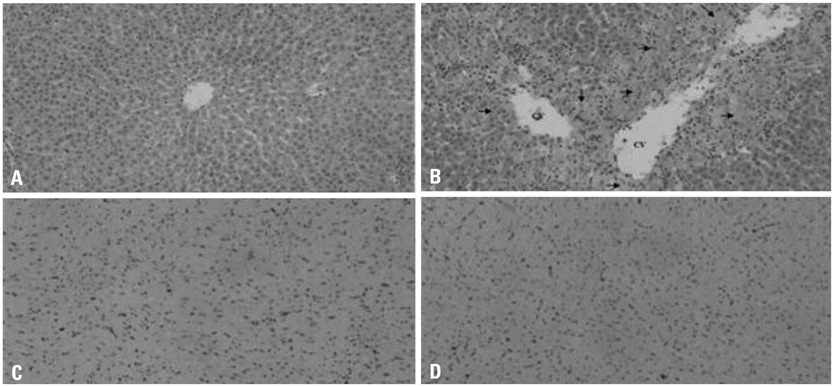
Following treatment, serum biochemical parameters including aspartate aminotransferase and alanine aminotransferase were elevated. Histopathological alterations of necrosis were observed in the liver, but not in the heart. However, alterations in gene expression were observed in both the liver and heart 24 hours after dosing. Transcriptional profiling revealed that acetaminophen changed the expression of genes implicated in oxidative stress, inflammatory processes, and apoptosis in the heart as well as in the liver. The numbers of up-regulated and down-regulated genes in the heart were 271 and 81, respectively, based on a two-fold criterion.
CONCLUSION
The induced expression of genes implicated in oxidative stress and inflammatory processes in the myocardium reflects molecular levels of injury caused by acetaminophen (APAP), which could not be identified by conventional histopathology.
Keyword
MeSH Terms
Figure
Reference
-
1. Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009. 39:11–21.
Article2. Moore M, Thor H, Moore G, Nelson S, Moldéus P, Orrenius S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J Biol Chem. 1985. 260:13035–13040.
Article3. Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med. 2010. 77:19–27.
Article4. Bronstein AC, Spyker DA, Cantilena LR Jr, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS). Clin Toxicol (Phila). 2007. 45:815–917.
Article5. Priyadarsiny P, Khattar SK, Malik R, Udupa V, Seshaiah A, Rahman S, et al. Differential gene expression analysis of a known hepatotoxin, N-acetyl-p-amino-phenol (APAP) as compared to its non-toxic analog, N-acetyl-m-amino-phenol (AMAP) in mouse liver. J Toxicol Sci. 2008. 33:163–173.
Article6. Jeong SY, Lim JS, Park HJ, Cho JW, Rana SV, Yoon S. Effects of acetaminophen on hepatic gene expression in mice. Physiol Chem Phys Med NMR. 2006. 38:77–83.7. Pimstone BL, Uys CJ. Liver necrosis and myocardiopathy following paracetamol overdosage. S Afr Med J. 1968. 42:259–262.8. Sanerkin NG. Acute myocardial necrosis in paracetamol poisoning. Br Med J. 1971. 3:478.
Article9. Zordoky BN, El-Kadi AO. H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. J Pharmacol Toxicol Methods. 2007. 56:317–322.
Article10. Thum T, Borlak J. Gene expression in distinct regions of the heart. Lancet. 2000. 355:979–983.
Article11. Fukushima T, Hamada Y, Yamada H, Horii I. Changes of micro-RNA expression in rat liver treated by acetaminophen or carbon tetrachloride--regulating role of micro-RNA for RNA expression. J Toxicol Sci. 2007. 32:401–409.
Article12. Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol. 2002. 181:106–115.
Article13. Reilly TP, Bourdi M, Brady JN, Pise-Masison CA, Radonovich MF, George JW, et al. Expression profiling of acetaminophen liver toxicity in mice using microarray technology. Biochem Biophys Res Commun. 2001. 282:321–328.
Article14. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010. 102:14–25.
Article15. Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010. 21:253–262.
Article16. Kröger A, Köster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002. 22:5–14.17. Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001. 19:623–655.
Article18. Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol. 2002. 39:499–508.
Article19. Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999. 85:199–207.
Article20. Liu J, Li C, Waalkes MP, Clark J, Myers P, Saavedra JE, et al. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology. 2003. 37:324–333.
Article21. Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part II. Their association with conventional and emerging risk factors, acute coronary events and cardiovascular risk prediction. Intern Med J. 2003. 33:450–462.
Article22. Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000. 28:1379–1386.
Article23. Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999. 66:876–888.
Article24. Benson V, McMahon AC, Lowe HC. ICAM-1 in acute myocardial infarction: a potential therapeutic target. Curr Mol Med. 2007. 7:219–227.
Article25. Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007. 93:903–907.
Article26. Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008. 27:6194–6206.
Article27. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009. 10:241–247.
Article28. Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008. 223:20–38.
Article29. Cuevas P, Carceller F, Giménez-Gallego G. Fibroblast growth factors in myocardial ischemia / reperfusion injury and ischemic preconditioning. J Cell Mol Med. 2001. 5:132–142.
Article30. Cuevas P, Carceller F, Martinez-Coso V, Cuevas B, Fernandez-Ayerdi A, Reimers D, et al. Cardioprotection from ischemia by fibroblast growth factor: role of inducible nitric oxide synthase. Eur J Med Res. 1999. 4:517–524.31. Fukushima T, Kikkawa R, Hamada Y, Horii I. Genomic cluster and network analysis for predictive screening for hepatotoxicity. J Toxicol Sci. 2006. 31:419–432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 4-Methylpyrazole for Acetaminophen Hepatotoxicity in a Rat Model
- Ethanol prevents from acetaminophen inducible hepatic necrosis by inhibiting its metabolic activation in mice
- Acetaminophen Induced Cytotoxicity and Altered Gene Expression in Cultured Cardiomyocytes of H9C2 Cells
- Comparison of tramadol/acetaminophen and codeine/acetaminophen/ibuprofen in onset of analgesia and analgesic efficacy for postoperative acute pain
- Mechanisms of Cardioprotection to Oxygen Radicals Induced by Ischemic Preconditioning in Isolated Perfused Rat Heart