Yonsei Med J.
2011 Mar;52(2):293-300. 10.3349/ymj.2011.52.2.293.
Efficacy of Itraconazole Prophylaxis for Autologous Stem Cell Transplantation in Children with High-Risk Solid Tumors: A Prospective Double-Blind Randomized Study
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kwsped@skku.edu
- 2Department of Pharmacy, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1779666
- DOI: http://doi.org/10.3349/ymj.2011.52.2.293
Abstract
- PURPOSE
The risk of invasive fungal infection is greater for allogeneic hematopoietic stem cell transplantation (HSCT) than for autologous transplantation. Therefore, many transplantation centers use antifungal prophylaxis for allogeneic HSCT, however, there exists no standard guidelines or consensus regarding autologous HSCT.
MATERIALS AND METHODS
A prospective double-blind randomized study was conducted in autologous HSCT recipients who were divided into prophylaxis and empirical treatment groups, and we investigated the efficacy of itraconazole prophylaxis in pediatric autologous HSCT.
RESULTS
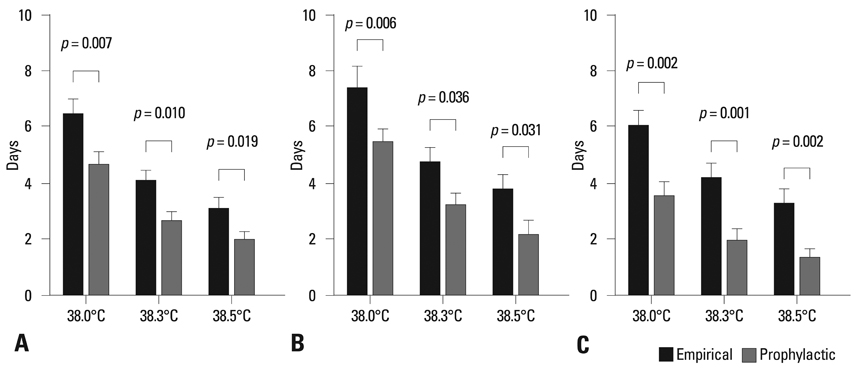
Total 87 autologous HSCT episodes in 55 children with high-risk solid tumors were studied. No invasive fungal infections occurred in either group. However, patients in the prophylaxis group had a significantly shorter duration of fever (p < 0.05) and received antibacterial treatment of shorter duration (p < 0.05) with fewer numbers of antibiotics (p < 0.05 for the use of second line antibiotics) than those in the empirical group. No significant additional adverse events were found with itraconazole prophylaxis.
CONCLUSION
Although beneficial effects such as a shorter duration of fever and reduced need for antibiotic use were observed in the prophylaxis group, the results were not sufficient to draw a definite recommendation about the routine use of antifungal prophylaxis in pediatric autologous HSCT recipients with high-risk solid tumors (Trial registration: NCT00336531).
MeSH Terms
-
Anti-Bacterial Agents/therapeutic use
Antifungal Agents/*therapeutic use
Child, Preschool
Cost-Benefit Analysis
Double-Blind Method
Hematopoietic Stem Cell Transplantation/*adverse effects
Humans
Itraconazole/*therapeutic use
Mycoses/*prevention & control
Neoplasms/*surgery
Prospective Studies
Risk Factors
Transplantation, Autologous
Treatment Outcome
Figure
Reference
-
1. Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992. 326:845–851.
Article2. Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study. J Infect Dis. 1995. 171:1545–1552.
Article3. Centers for Disease Control and Prevention. Infectious Diseases Society of America. American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2000. 6:659–713. 715717–727. quiz 729-33.4. Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002. 34:730–751.
Article5. Centers for Disease Control and Prevention. Infectious Disease Society of America. American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000. 49:1–125. CE1-7.6. Morgenstern GR, Prentice AG, Prentice HG, Ropner JE, Schey SA, Warnock DW. U.K. Multicentre Antifungal Prophylaxis Study Group. A randomized controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies. Br J Haematol. 1999. 105:901–911.
Article7. Vardakas KZ, Michalopoulos A, Falagas ME. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol. 2005. 131:22–28.
Article9. Baddley JW, Stroud TP, Salzman D, Pappas PG. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001. 32:1319–1324.
Article10. Cornet M, Fleury L, Maslo C, Bernard JF, Brücker G. Invasive Aspergillosis Surveillance Network of the Assistance Publique-Hôpitaux de Paris. Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. J Hosp Infect. 2002. 51:288–296.
Article11. Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003. 102:827–833.
Article12. Grow WB, Moreb JS, Roque D, Manion K, Leather H, Reddy V, et al. Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002. 29:15–19.
Article13. Jantunen E, Salonen J, Juvonen E, Koivunen E, Siitonen T, Lehtinen T, et al. Invasive fungal infections in autologous stem cell transplant recipients: a nation-wide study of 1188 transplanted patients. Eur J Haematol. 2004. 73:174–178.
Article14. Kojima R, Kami M, Nannya Y, Kusumi E, Sakai M, Tanaka Y, et al. Incidence of invasive aspergillosis after allogeneic hematopoietic stem cell transplantation with a reduced-intensity regimen compared with transplantation with a conventional regimen. Biol Blood Marrow Transplant. 2004. 10:645–652.
Article15. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002. 34:909–917.
Article16. Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005. 43:Suppl 1. S49–S58.
Article17. Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics. 2006. 117:e711–e716.
Article18. Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007. 45:1161–1170.
Article19. Post MJ, Lass-Floerl C, Gastl G, Nachbaur D. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl Infect Dis. 2007. 9:189–195.
Article20. Castagnola E, Cesaro S, Giacchino M, Livadiotti S, Tucci F, Zanazzo G, et al. Fungal infections in children with cancer: a prospective, multicenter surveillance study. Pediatr Infect Dis J. 2006. 25:634–639.21. Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005. 36:621–629.
Article22. Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O'Brien MR, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005. 11:362–370.
Article23. Benjamin DK Jr, Miller WC, Bayliff S, Martel L, Alexander KA, Martin PL. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J. 2002. 21:227–234.
Article24. George B, Mathews V, Viswabandya A, Srivastava A, Chandy M. Infections in children undergoing allogeneic bone marrow transplantation in India. Pediatr Transplant. 2006. 10:48–54.
Article25. Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 2000. 26:999–1004.
Article26. Lehrnbecher T, Becker M, Schwabe D, Köhl U, Kriener S, Hunfeld KP, et al. Primary intestinal aspergillosis after high-dose chemotherapy and autologous stem cell rescue. Pediatr Infect Dis J. 2006. 25:465–466.
Article27. Jathavedam A, Feldman DR, Ishill N, Turkula S, Patil S, Bosl GJ, et al. Infectious complications from high-dose chemotherapy and autologous stem cell transplantation for metastatic germ cell tumors. Biol Blood Marrow Transplant. 2008. 14:595–600.
Article28. Simon A, Besuden M, Vezmar S, Hasan C, Lampe D, Kreutzberg S, et al. Itraconazole prophylaxis in pediatric cancer patients receiving conventional chemotherapy or autologous stem cell transplants. Support Care Cancer. 2007. 15:213–220.
Article29. Cordonnier C, Ribaud P, Herbrecht R, Milpied N, Valteau-Couanet D, Morgan C, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006. 42:955–963.
Article30. Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007. 25:5471–5489.
Article31. Castagnola E, Faraci M, Moroni C, Bandettini R, Granata C, Caruso S, et al. Invasive mycoses in children receiving hemopoietic SCT. Bone Marrow Transplant. 2008. 41:Suppl 2. S107–S111.
Article32. Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007. 356:348–359.
Article33. Abdel-Rahman SM, Jacobs RF, Massarella J, Kauffman RE, Bradley JS, Kimko HC, et al. Single-dose pharmacokinetics of intravenous itraconazole and hydroxypropyl-beta-cyclodextrin in infants, children, and adolescents. Antimicrob Agents Chemother. 2007. 51:2668–2673.
Article34. Willems L, van der Geest R, de Beule K. Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics. J Clin Pharm Ther. 2001. 26:159–169.
Article35. Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006. 26:1169–1174.
Article36. Endimiani A, Perez F, Bonomo RA. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 2008. 6:805–824.
Article37. Capparelli FJ, Diaz MF, Hlavnika A, Wainsztein NA, Leiguarda R, Del Castillo ME. Cefepime- and cefixime-induced encephalopathy in a patient with normal renal function. Neurology. 2005. 65:1840.
Article38. Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis. 2004. 8:59–61.
Article39. Schonfeld W, Wang Cheng J, Tong KB, Seifeldin R. Cost-effectiveness analysis of antifungal prophylaxis in patients undergoing hematopoietic stem cell transplantation. Clin Ther. 2008. 30:964–973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Cefepime Prophylaxis for Autologous Stem Cell Transplantation in Children with High-risk Solid Tumor
- Prospective Randomized Comparative Observations of Infectious Complications with or without Antimicrobial Prophylaxis, during Autologous Stem Cell Transplantation
- High-dose chemotherapy and autologous stem cell transplantation for pediatric brain tumors
- A Case of Autologous Cord Blood Stem Cell Transplantationin Stage IV Neuroblastoma
- A Case of Scleroderma following Autologous Peripheral Stem Cell Transplantation