Yonsei Med J.
2006 Aug;47(4):558-567. 10.3349/ymj.2006.47.4.558.
Implication of ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 in Implantational Remodeling of a Mouse Uterus
- Affiliations
-
- 1Department of Biotechnology, College of Natural Sciences, Seoul Women's University, Seoul, Korea. hwkim@swu.ac.kr
- 2Department of Applied Microbiology, College of Natural Sciences, Seoul Women's University, Seoul, Korea.
- 3School of Biotechnology and Biomedical Science, Inje University, Kimhae, Korea.
- 4Department of Obstetrics and Gynecology, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 1779507
- DOI: http://doi.org/10.3349/ymj.2006.47.4.558
Abstract
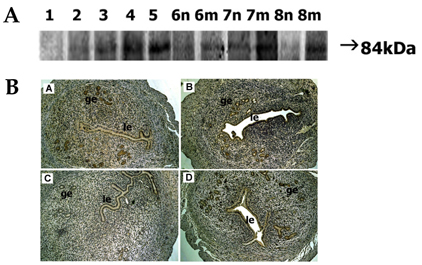
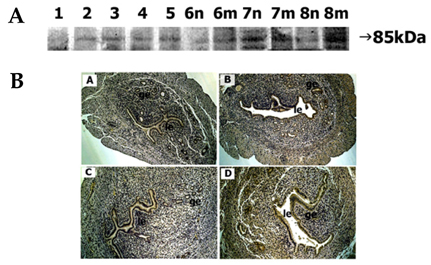
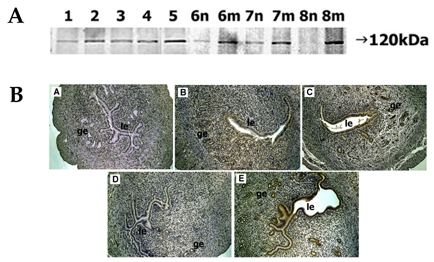
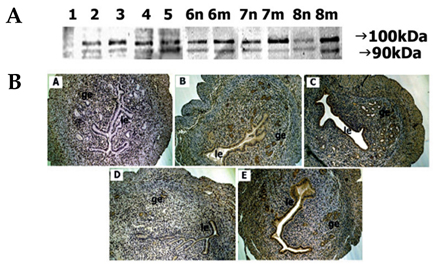
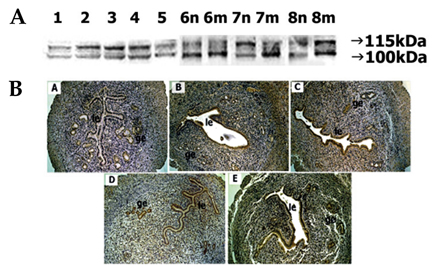
- In the present study, whether the ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 proteins might play a role in mouse uterus during periimplantation period was investigated. Immunoblotting analyses demonstrated that all ADAM proteins consistently appeared throughout days 1 to 8 of pregnancy but with a variation depending on the species of ADAM gene, the progression of pregnancy, and the site of the uterus. Immunohistochemical analyses indicated that ADAM proteins were localized in the luminal or glandular epithelial layers with a varying intensity depending on the species of ADAM and the progression of pregnancy. Particularly ADAM-8, -12, and -15, were predominantly located in the implantation site of the uterine tissues, whereas little or no protein was localized in the interimplantation site. Based upon these observations, it is suggested that the ADAMs might play an important role in the remodeling of the mouse uterus during the periimplantation period.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhou M, Graham R, Russell G, Croucher PI. MDC-9 (ADAM-9/Meltrin gamma) functions as an adhesion molecule by binding the alpha(v)beta(5) integrin. Biochem Biophys Res Commun. 2001. 280:574–580.2. Zhao Z, Gruszczynska-Biegala J, Cheuvront T, Yi H, von der Mark H, von der Mark K, et al. Interaction of the disintegrin and cysteine-rich domains of ADAM12 with integrin alpha7beta1. Exp Cell Res. 2004. 298:28–37.3. Eto K, Puzon-McLaughlin W, Sheppard DJ, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000. 275:34922–34930.4. Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, et al. ADAMTS-1, a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000. 105:1345–1352.5. Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999. 274:25555–25563.6. Blankenship TN, Given RL. Loss of laminin and type IV collagen in uterine luminal epithelial basement membranes during blastocyst implantation in the mouse. Anat Rec. 1995. 243:27–36.7. Greca CD, Nader HB, Dietrich CP, Abrahamsohn PA, Zorn TM. Ultrastructural cytochemical characterization of collagen-associated proteoglycans in the endometrium of mice. Anat Rec. 2000. 259:413–423.8. San Martin S, Soto-Suazo M, Zorn TM. Perlecan and syndecan-4 in uterine tissues during the early pregnancy in mice. Am J Reprod Immunol. 2004. 52:53–59.9. Curry TE Jr, Osteen KG. The matrix metalloproteinase system, changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003. 24:428–465.10. Kim J, Kim H, Lee SJ, Chio YM, Lee SJ, Lee JY. Abundance of ADAM-8, -9, -10, -12, -15, -17 and ADAMTS-1 in mouse uterus during the oestrous cycle. Reprod Fertil Dev. 2005. 17:543–555.11. Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003. 278:30469–30477.12. Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156), implications for neuron-glia interactions during neurodegeneration. J Neurosci. 2000. 20:7964–7971.13. Choi SJ, Han JH, Roodman GD. ADAM8, a novel osteoclast stimulating factor. J Bone Miner Res. 2001. 16:814–822.14. Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, et al. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002. 277:48210–48219.15. Kadota N, Suzuki A, Nakagami Y, Izumi T, Endo T. Endogenous meltrin alpha is ubiquitously expressed and associated with the plasma membrane but exogenous meltrin alpha is retained in the endoplasmic reticulum. J Biochem (Tokyo). 2000. 128:941–949.16. Kawaguchi N, Sundberg C, Kveiborg M, Moghadaszadeh B, Asmar M, Dietrich N, et al. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J Cell Sci. 2003. 116:3893–3904.17. Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun. 2000. 278:511–515.18. Lum L, Reid MS, Blobel CP. Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J Biol Chem. 1998. 273:26236–26247.19. Martin J, Eyestone LV, Davies M, Williams JD, Steadman R. The role of ADAM 15 in glomerular mesangial cell migration. J Biol Chem. 2002. 277:33683–33689.20. Olson GE, Winfrey VP, Matrisian PE, NagDas SK, Hoffman LH. Blastocyst-dependent upregulation of metalloproteinase/disintegrin MDC9 expression in rabbit endometrium. Cell Tissue Res. 1998. 293:489–498.21. Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003. 278:3386–3394.22. Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, et al. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995. 136:3639–3647.23. Schwettmann L, Tschesche H. Cloning and expression in Pichia pastoris of metalloprotease domain of ADAM 9 catalytically active against fibronectin. Protein Expr Purif. 2001. 21:65–70.24. Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004. 164:769–779.25. Srour N, Lebel A, McMahon S, Fournier I, Fugere M, Day R, et al. TACE/ADAM-17 maturation and activation of sheddase activity require proprotein convertase activity. FEBS Lett. 2003. 554:275–283.26. Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003. 17:7–30.27. Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003. 278:23656–23665.28. Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, et al. ADAMTS-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004. 70:1096–1105.29. San Martin S, Soto-Suazo M, Zorn TM. Distribution of versican and hyaluronan in the mouse uterus during decidualization. Braz J Med Biol Res. 2003. 36:1067–1071.30. Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, et al. Progesterone-regulated genes in the ovulation process, ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000. 97:4689–4694.31. Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K, et al. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn. 2005. 232:221–231.32. Weskamp G, Cai H, Brodie TA, Higashyama S, Manova K, Ludwig T, et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002. 22:1537–1544.33. Kurisaki T, Masuda A, Sudo K, Sakagami J, Higashiyama S, Matsuda Y, et al. Phenotypic analysis of Meltrin alpha (ADAM12)-deficient mice, involvement of Meltrin alpha in adipogenesis and myogenesis. Mol Cell Biol. 2003. 23:55–61.34. Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie T, et al. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003. 23:5614–5624.35. Hanakawa Y, Matsuyoshi N, Stanley JR. Expression of desmoglein 1 compensates for genetic loss of desmoglein 3 in keratinocyte adhesion. J Invest Dermatol. 2002. 119:27–31.36. Owada Y, Suzuk I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol Cell Biochem. 2002. 239:83–86.37. Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002. 21:611–621.38. Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1-deficient mice. Endocrinology. 1997. 138:4902–4911.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of ADAM-8, 9, 10, 12, 15, 17 and ADAMTS-1 Genes in Mouse Uterus During Periimplantation Period
- Frequency of Pro475Ser Polymorphism of ADAMTS13 Gene and Its Association with ADAMTS-13 Activity in the Korean Population
- Interrelation between Expression of ADAM 10 and MMP 9 and Synthesis of Peroxynitrite in Doxorubicin Induced Cardiomyopathy
- Changes in VWF-cleaving Metalloprotease (ADAMTS 13) activity in the thrombotic microangiopathy after kidney tranplantation
- Thrombotic thrombocytopenic purpura with decreased level of ADAMTS-13 activity and increased level of ADAMTS-13 inhibitor in an adolescent