Yonsei Med J.
2006 Aug;47(4):466-474. 10.3349/ymj.2006.47.4.466.
Nine-Year Survival of Lymphoblastic Lymphoma Patients
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. medi@yumc.yonsei.ac.kr
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1779494
- DOI: http://doi.org/10.3349/ymj.2006.47.4.466
Abstract
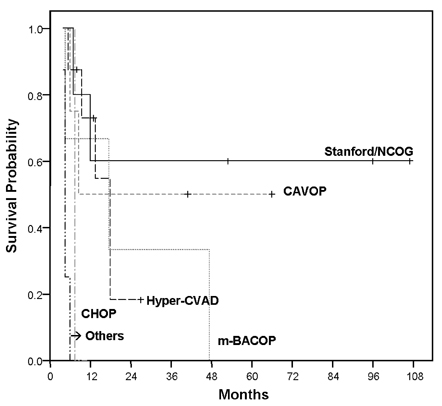
- This study aimed to analyze the overall survival period of adult lymphoblastic lymphoma patients treated with various therapeutic regimens, and to assess the determinants affecting survival outcome. Twenty-five adult patients with lymphoblastic lymphoma who had been treated at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea from June 1996 to June 2005 were analyzed retrospectively. As an initial remission induction chemotherapy, the hyper-CVAD regimen was performed in eight patients, the Stanford/Northern California Oncology Group (NCOG) regimen in five, the CAVOP regimen in four, the m-BACOP regimen in three, and the CHOP regimen in one patient. Patients were divided into two groups according to their therapeutic modalities. Twenty patients received conventional chemotherapy alone and five received subsequent PBSCT after conventional chemotherapy. Four patients of the PBSCT group underwent autologous PBSCT and one underwent allogeneic PBSCT. The overall response rate was 80% (60% showing a complete response, 20% showing a partial response) and the relapse rate was 73.3%. The overall survival (OS) rate was 55.1% at 1 year, 31.5% at 5 years, and 23.6% at 9 years. The disease-free survival (DFS) rate was 46.7% at 1 year and 30.0% at 7 years. The 5-year OS rate in relation to the regimens was 60% with the Stanford/NCOG regimen, 50% with the CAVOP regimen, and 33.3% with the m-BACOP regimen. The patients treated with the hyper-CVAD regimen had an 18.2% 2-year OS rate, and other patients with CHOP or COPBLAM-V expired early in their course. The OS rate in patients treated with conventional chemotherapy alone was 19.8%, whereas patients treated with subsequent PBSCT after chemotherapy showed 50% overall survival (p=0.25). The age at presentation influenced the outcome of the patients (p=0.01). The Stanford/NCOG regimen is an effective initial choice of therapy for lymphoblastic lymphoma patients, and is superior to the hyper-CVAD regimen in complete response rate and overall survival rate (p=0.36). Addition of PBSCT after chemotherapy may be needed for achieving optimal outcomes.
Keyword
MeSH Terms
Figure
Reference
-
1. Picozzi VJ Jr, Coleman CN. Lymphoblastic lymphoma. Semin Oncol. 1990. 17:96–103.2. Coleman CN, Picozzi VJ Jr, Cox RS, McWhirter K, Weiss LM, Cohen JR, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol. 1986. 4:1628–1637.3. Thomas DA, O'Brien S, Cortes J, Giles FJ, Fanderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004. 104:1624–1630.4. Sweetham JW, Santini G, Qian W, Guelfi M, Schmitz N, Simnett S, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/ maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol. 2001. 19:2927–2936.5. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971. 31:1860–1861.6. Hahn JS, Lee S, Chong SY, Min YH, Ko YW. Eight-year experience of malignant lymphoma-survival and prognostic factors. Yonsei Med J. 1997. 38:270–284.7. Hahn JS, Kim YS, Lee YC, Yang WI, Lee SY, Suh CO. Eleven-year experience of low grade lymphoma in Korea (Based on REAL classification). Yonsei Med J. 2003. 44:757–770.8. Chen YC, Ho CL, Kao WY, Hwang JM, Sheu LF, Chao TY. Adult lymphoblastic lymphoma in Taiwan: an analysis of treatment results of 26 patients. Ann Hematol. 2001. 80:647–652.9. Levine JE, Harris RE, Loberiza FR Jr, Armitage JO, Vose JM, Van Besien K, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood. 2003. 101:2476–2482.10. Kim DW, Sun JM, Kwon JH, Oh DY, Lee JJ, Jo YH, et al. An analysis of treatment results of lymphoblastic lymphoma in adults according to the chemotherapy regimens. Korean J Hematol. 2003. 38:32–39.11. Oh SJ, Kim SB, Suh CW, Ahn JH, Kim WK, Lee JS. Feasibility of autologous stem cell transplantation in 12 patients with adult lymphoblastic lymphoma; Experience of Single Institution (Abstract). 2002. In : The 28th Annual Meeting of The Korean Cancer Association;12. Le Gouill S, Lepretre S, Briere J, Morel P, Bouabdallah R, Raffoux E, et al. Adult lymphoblastic lymphoma: a retrospective analysis of 92 patients under 61 years included in the LNH87/93 trials. Leukemia. 2003. 17:2220–2224.13. Smith SM, Grinblatt D, van Besien K. Autologous and allogeneic transplantation for aggressive NHL. Cytotherapy. 2002. 4:223–240.14. Bierman PJ. Allogeneic bone marrow transplantation for lymphoma. Blood Rev. 2000. 14:1–13.15. Lee KH. Allogeneic HCT for lymphoma. Korean J Hematol. 2005. 40:Suppl 1. S22.16. Hahn JS, Cho JY, Lee ST, Chung SY, Min YH, Ko YW. Therapeutic outcome and prognosis in elderly patients with non-Hodgkin's lymphoma. J Korean Cancer Assoc. 1999. 31:320–330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Non-T,Non-B Primary Cutaneous Lymphoblastic Lymphoma
- Solitary Lymphoblastic Lymphoma of the Thoracic Spine
- A Case of B-Lymphoblastic Leukemia/Lymphoma Detected as a Laterally Spreading Tumor
- Cutaneous Involvement of T-cell Lymphoblastic Lymphoma
- Rapid Progression of Mediastinal Tumor within a Few Days: A Case Report of T Cell Lymphoblastic Lymphoma