J Korean Med Sci.
2013 Dec;28(12):1801-1806. 10.3346/jkms.2013.28.12.1801.
Antibodies to Interferon beta in Patients with Multiple Sclerosis Receiving CinnoVex, Rebif, and Betaferon
- Affiliations
-
- 1Deparment of Immunology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
- 2Deparment of Immunology, School of Medicine and Applied Physiology Research Center, Isfahan University of Medical Sciences, Isfahan and Department of Biology, Faculty of Sciences, University of Isfahan, Isfahan, Iran. s.h.zarkesh@sheffield.ac.uk
- 3Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
- KMID: 1779423
- DOI: http://doi.org/10.3346/jkms.2013.28.12.1801
Abstract
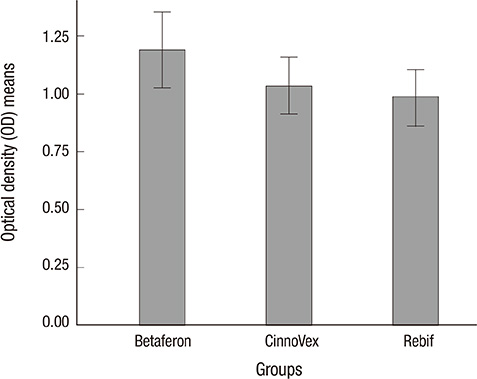
- Treatment with interferon beta (IFN-beta) induces the production of binding antibodies (BAbs) and neutralizing antibodies (NAbs) in patients with multiple sclerosis (MS). NAbs against IFN-beta are associated with a loss of IFN-beta bioactivity and decreased clinical efficacy of the drug. The objective of this study was to evaluate the incidence and the prevalence of binding antibodies (BAbs) and neutralizing antibodies (NAbs) to IFN-beta in MS patients receiving CinnoVex, Rebif, or Betaferon. The presence of BAbs was studied in serum samples from 124 MS patients using one of these IFN-beta medications by ELISA. The NAbs against IFN-beta were measured in BAb-positive MS patients receiving IFN-beta using an MxA gene expression assay (real-time RT-PCR). Of the 124 patients, 36 (29.03%) had BAbs after at least 12 months of IFN-beta treatment. The proportion of BAb+ was 38.1% for Betaferon, 21.9% for Rebif, and 26.8% for CinnoVex. Five BAb-positive MS patients were lost to follow-up; thus 31 BAb-positive MS patients were studied for NAbs. NAbs were present in 25 (80.6%) of BAb-positive MS patients receiving IFN-beta. In conclusion, the three IFN-beta preparations have different degrees of immunogenicity.
Keyword
MeSH Terms
-
Adolescent
Adult
Antibodies/*blood/immunology
Antibodies, Neutralizing/*blood/immunology
Cross Reactions
DNA, Complementary/metabolism
Enzyme-Linked Immunosorbent Assay
Female
Humans
Interferon-beta/*immunology/therapeutic use
Male
Middle Aged
Multiple Sclerosis/drug therapy/*immunology
Myxovirus Resistance Proteins/genetics
Reverse Transcriptase Polymerase Chain Reaction
Young Adult
Antibodies
Antibodies, Neutralizing
DNA, Complementary
Interferon-beta
Myxovirus Resistance Proteins
Figure
Reference
-
1. Van Baarsen LG, Vosslamber S, Tijssen M, Baggen JM, van der Voort LF, Killestein J, van der Pouw Kraan TC, Polman CH, Verweij CL. Pharmacogenomics of interferon-beta therapy in multiple sclerosis: baseline IFN signature determines pharmacological differences between patients. PLoS One. 2008; 3:e1927.2. Pachner A, Narayan K, Price N, Hurd M, Dail D. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn. 2003; 7:17–25.3. Hartung HP, Munschauer F 3rd, Schellekens H. Significance of neutralizing antibodies to interferon beta during treatment of multiple sclerosis: expert opinions based on the Proceedings of an International Consensus Conference. Eur J Neurol. 2005; 12:588–601.4. Bermel RA, Rudick RA. Interferon-beta treatment for multiple sclerosis. Neurotherapeutics. 2007; 4:633–646.5. Bertolotto A, Gilli F, Sala A, Audano L, Castello A, Magliola U, Melis F, Giordana MT. Evaluation of bioavailability of three types of IFNbeta in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods. 2001; 256:141–152.6. Bertolotto A, Gilli F, Sala A, Capobianco M, Malucchi S, Milano E, Melis F, Marnetto F, Lindberg RL, Bottero R, et al. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003; 60:634–639.7. Cohen BA, Oger J, Gagnon A, Giovannoni G. The implications of immunogenicity for protein-based multiple sclerosis therapies. J Neurol Sci. 2008; 275:7–17.8. McKay F, Schibeci S, Heard R, Stewart G, Booth D. Analysis of neutralizing antibodies to therapeutic interferon-beta in multiple sclerosis patients: a comparison of three methods in a large Australasian cohort. J Immunol Methods. 2006; 310:20–29.9. Deisenhammer F, Schellekens H, Bertolotto A. Measurement of neutralizing antibodies to interferon beta in patients with multiple sclerosis. J Neurol. 2004; 251:II31–II39.10. Sominanda A, Hillert J, Fogdell-Hahn A. In vivo bioactivity of interferon-beta in multiple sclerosis patients with neutralising antibodies is titre-dependent. J Neurol Neurosurg Psychiatry. 2008; 79:57–62.11. Namaka M, Pollitt-Smith M, Gupta A, Klowak M, Vasconcelos M, Turcotte D, Gong Y, Melanson M. The clinical importance of neutralizing antibodies in relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2006; 22:223–239.12. Fernández O, Mayorga C, Luque G, Guerrero M, Guerrero R, Leyva L, León A, Blanca M. Study of binding and neutralising antibodies to interferon-beta in two groups of relapsing-remitting multiple sclerosis patients. J Neurol. 2001; 248:383–388.13. Cutrone EC, Langer JA. Identification of critical residues in bovine IFNAR-1 responsible for interferon binding. J Biol Chem. 2001; 276:17140–17148.14. Meager A, Dolman C, Dilger P, Bird C, Giovannoni G, Schellekens H, Thorpe R, Wadhwa M. An assessment of biological potency and molecular characteristics of different innovator and noninnovator interferon-beta products. J Interferon Cytokine Res. 2011; 31:383–392.15. Kivisäkk P, Alm GV, Fredrikson S, Link H. Neutralizing and binding anti-interferon-beta (IFN-beta) antibodies: a comparison between IFN-beta-1a and IFN-beta-1b treatment in multiple sclerosis. Eur J Neurol. 2000; 7:27–34.16. Monzani F, Meucci G, Caraccio N, Saviozzi M, Casolaro A, Moscato G, Lombardo F, Mosti S, Scagnolari C, Bruschi F, et al. Discordant effect of IFN-beta1a therapy on anti-IFN antibodies and thyroid disease development in patients with multiple sclerosis. J Interferon Cytokine Res. 2002; 22:773–781.17. Perini P, Calabrese M, Biasi G, Gallo P. The clinical impact of interferon beta antibodies in relapsing-remitting MS. J Neurol. 2004; 251:305–309.18. Gilli F, Marnetto F, Caldano M, Sala A, Malucchi S, Capobianco M, Bertolotto A. Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler. 2006; 12:47–57.19. Sominanda A, Rot U, Suoniemi M, Deisenhammer F, Hillert J, Fogdell-Hahn A. Interferon beta preparations for the treatment of multiple sclerosis patients differ in neutralizing antibody seroprevalence and immunogenicity. Mult Scler. 2007; 13:208–214.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of childhood relapsing/remitting multiple sclerosis and interferon beta-1b treatment in a Korean patient
- Reduction of Disease Activity in Patient with Relapsing-Remitting Multiple Sclerosis after Switching to Teriflunomide from Interferon Beta
- Interferon beta-1b Treatment in a Korean Girl with Multiple Sclerosis
- Interferon-beta Induced Skin Necrosis
- Chronic Inflammatory Demyelinating Polyneuropathy Developed during Interferon-beta Therapy in a Patient with Multiple Sclerosis