J Korean Med Sci.
2009 Jun;24(3):474-480. 10.3346/jkms.2009.24.3.474.
EC-18, a Synthetic Monoacetyldiacylglyceride, Inhibits Hematogenous Metastasis of KIGB-5 Biliary Cancer Cell in Hamster Model
- Affiliations
-
- 1Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 2Department of Oncology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Asan Institute for Life Sciences, Seoul, Korea.
- 4R & D Center, EnzyChem Co. Ltd, Daejon, Korea.
- 5Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. sglee2@amc.seoul.kr
- 6Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 1779166
- DOI: http://doi.org/10.3346/jkms.2009.24.3.474
Abstract
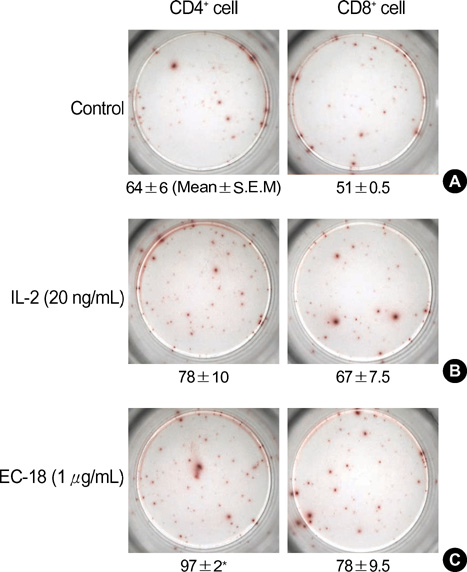
- EC-18 (monoacetyldiacylglyceride) stimulates T cell production of IL-2, IL-4, IL-12, IFN-gamma, and GM-CSF in vitro. To study the effects of these cytokines stimulated by EC-18 on cancer cells, we applied hamster biliary cancer model, a difficult cancer to treat. Cancer (KIGB-5) cells were given intravenously to produce hematogenous metastatic lung lesions which were treated with EC-18 at 10, 25, and 50 mg/kg/day respectively. The fourth group was untreated control. At 4th, 8th, and 12th week the lungs were examined. EC-18 treated groups showed only a few microscopic lung lesions and no evidence of metastatic lesion with highest dose whereas widespread gross lung lesions were observed in untreated control. To investigate whether the anti-tumor effect of EC-18 is associated with suppression of tumor cell Toll-like receptor 4 (TLR-4) expression in addition to stimulation of the immune cells, KIGB-5 cells were exposed to LPS with or without EC-18. TLR-4 mRNA and protein expression, measured by reverse transcriptase PCR (RT-PCR), real-time quantitative PCR and western blot analysis, showed suppression of TLR-4 expression in KIGB-5 cells treated with EC-18 compared with control. In conclusion, EC-18 has a significant anti-tumor effect in this experimental model of biliary cancer suggesting potential for clinical application to this difficult cancer.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) Modulates Th2 Immunity through Attenuation of IL-4 Expression
Sun Young Yoon, Ho Bum Kang, Young-Eun Ko, Su-Hyun Shin, Young-Jun Kim, Ki-Young Sohn, Yong-Hae Han, Saeho Chong, Jae Wha Kim
Immune Netw. 2015;15(2):100-109. doi: 10.4110/in.2015.15.2.100.Effect of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol on Immune Functions in Healthy Adults in a Randomized Controlled Trial
Hee-Jin Hwang, Ki-Young Sohn, Yong-Hae Han, Saeho Chong, Sun Young Yoon, Young-Jun Kim, Jinseoun Jeong, Sang-Hwan Kim, Jae Wha Kim
Immune Netw. 2015;15(3):150-160. doi: 10.4110/in.2015.15.3.150.
Reference
-
1. Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroentrology. 2005. 128:1655–1667.
Article2. Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004. 66:167–179.
Article3. Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cancer using high dose bolus interleukin-2. JAMA. 1994. 271:907–913.4. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull. 2004. 52:874–878.
Article5. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004. 27:1121–1125.
Article6. Kim MH, Lee SS, Lee SK, Lee SG, Suh CW, Gong GY, Park JS, Kim YH, Kim SH. Interleukin-2 gene-encoded stromal cells inhibit the growth of metastatic cholangiocarcinomas. World J Gastroenterol. 2006. 12:1889–1894.
Article7. Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005. 65:5009–5014.
Article8. Korean patent application. 10-2005-0065792. 2005. 07. 20.9. Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. DEV BIO. 2008. 315:72–88.
Article10. Hillman GG, Younes E, Visscher D, AIi E, Lam JS, Montecillo E, Pontes JE, Haas GP, Puri RK. Systemic treatment with interleukin-4 induces regression of pulmonary metastases in murine renal cell carcinoma model. Cell Immunol. 1995. 160:257–263.11. Peace DJ, Kern DE, Schultz KR, Greenberg PD, Cheever MA. IL-4-induced lymphokine-activated killer cells. Lytic activity is mediated by phenotypically distinct natural killer-like and T cell-like large granular lymphocytes. J Immunol. 1988. 140:3679–3685.12. Gambacorti-Passerini C, Rivoltini L, Supino R, Rodolfo M, Radrizzani M, Fossati G, Parmiani G. Susceptibility of chemoresistant murine and human tumor cells to lysis by interleukin-2 activated lymphocytes. Cancer Res. 1988. 48:2372–2376.13. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFN-gamma and lymphocytes prevent primary tumor development and shape tumor immunogenicity. Nature. 2001. 410:1107–1111.14. Egilmez NK, Hess SD, Chen FA, Takita H, Conway TF, Bankert RB. Human CD4+ effector T cells mediate indirect interleukin-12 and interferon-γ-dependent suppression of autologous HLA-negative lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2002. 62:2611–2617.15. Chuang M, Lee MW, Zhao D, Severson DL. Metabolism of a long chain diacylglycerol by permeabilized A10 smooth muscle cells. Am J Physiol. 1993. 265:C927–C933.16. Forcic D, Mazuran R. Modulation of [Ca2+]i in freshly isolated mouse lymphocytes with in vivo priming. Immunol Lett. 1999. 67:23–30.17. Randriamampita C, Trautmann A. Ca2+ signals and T-lymphocytes; "New mechanisms and functions in Ca2+ signaling". Biol Cell. 2004. 96:69–78.18. Han SY, Cho SH, Kim SY, Seo JT, Moon SJ, Jhon GJ. Monoacetyl-diglycerides as new Ca2+ mobilizing agents in rat pancreatic acinar cells. Bioorg Med Chem Lett. 1999. 9:59–64.19. Goni FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res. 1999. 38:1–48.20. Okamoto M, Sato M. Toll-like receptor signaling in anti-cancer immunity. J Med Invest. 2003. 50:9–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A predictive model for lymph node metastasis using tumor location in presumed early-stage endometrioid endometrial cancer patients
- EXPRESSION OF TGF-alpha AND TGF-beta
- The Establishment of Sperm Penetration Assay Using Cryopreserved Hamster Oocyte
- Significance of lymph node metastasis in the survival of stage IV colorectal cancer by hematogenous metastasis
- CircSMAD2 accelerates endometrial cancer cell proliferation and metastasis by regulating the miR-1277-5p/MFGE8 axis