Ann Surg Treat Res.
2018 Oct;95(4):201-212. 10.4174/astr.2018.95.4.201.
Significance of lymph node metastasis in the survival of stage IV colorectal cancer by hematogenous metastasis
- Affiliations
-
- 1Department of Surgery, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea.
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Center for Colorectal Cancer, National Cancer Center, Goyang, Korea.
- 5Department of Surgery, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea. heosc3@brmh.org
- KMID: 2421198
- DOI: http://doi.org/10.4174/astr.2018.95.4.201
Abstract
- PURPOSE
Although lymph node (LN) metastasis is an important prognostic marker of colorectal cancer (CRC), the effect of LN metastasis on the survival of stage IV CRC is debated yet.
METHODS
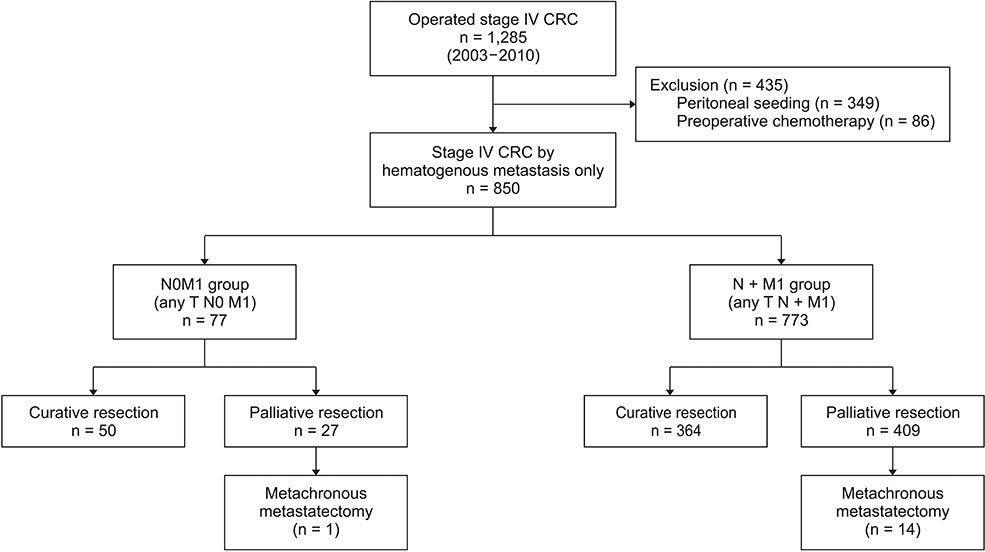
LN status and survivals as well as clinicopathological features of synchronous stage IV CRC patients, operated for 8 years, were analyzed. Patients with hematogenous metastases were included only but those with peritoneal seeding or preoperative adjuvant therapy were not included.
RESULTS
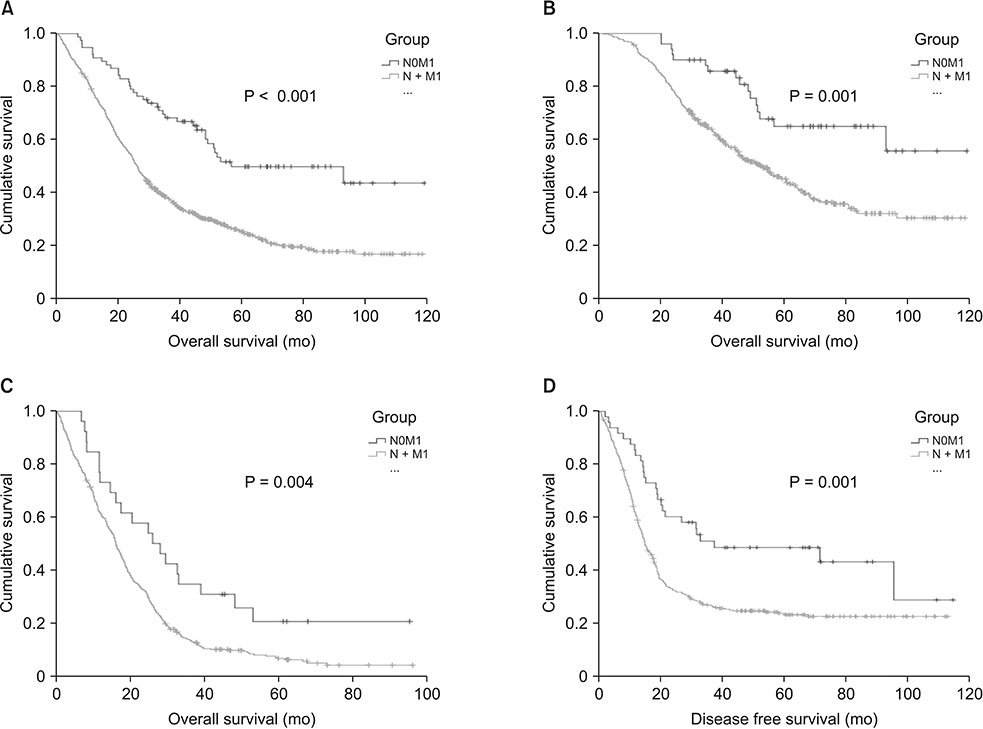
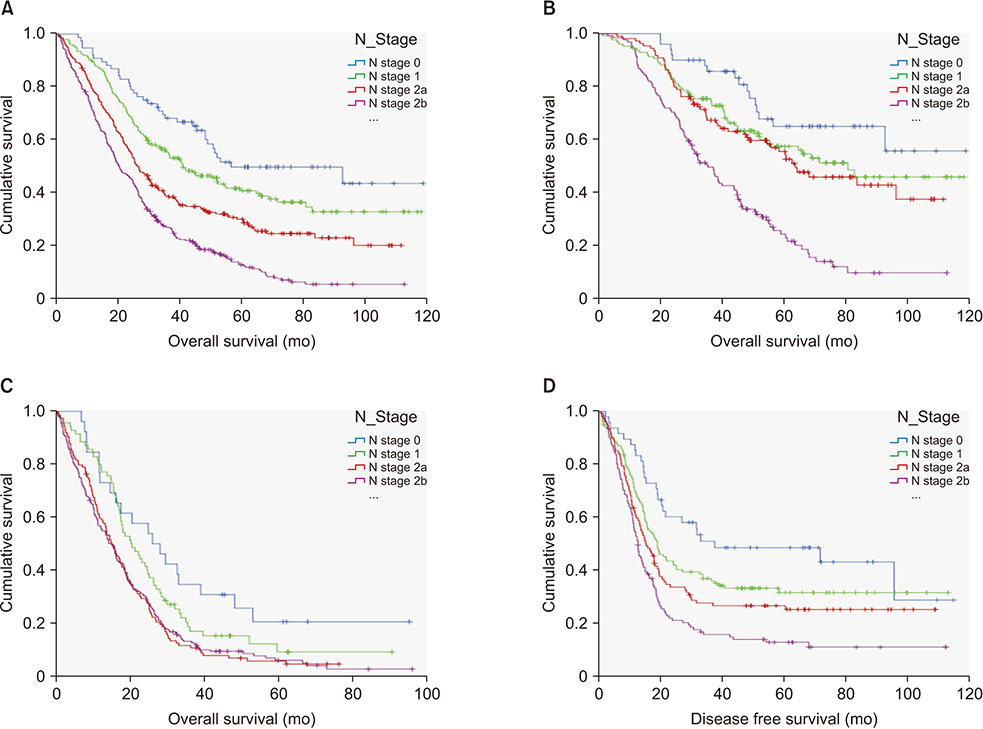
Total 850 patients were enrolled and 77 (9.1%) were without LN metastases (N0M1). N0M1 patients were older and have favorable pathological features including lower CEA than patients with LN metastasis (N + M1). The pathologically poor features accumulated with N stage progression within N + M1. N0M1 had better 5-year overall survival (OS) and disease free survival than N + M1. And 5-year OS's within N + M1 group were stratified and different according to N stage progression, although the effect of N stage progression is different according to curative resection or not. When compared with stage III, 5-year OS of N0M1 with curative resection was comparable to that of anyTN2aM0 and was better than anyTN2bM1.
CONCLUSION
LN metastasis is a significant prognostic factor in stage IV by hematogenous metastasis, too. N stage progression accumulates pathologically poor prognostic factors. However, the effect on survival of each N stage progression differs depending on curative resection or not of the hematogenous metastases.
Figure
Reference
-
1. Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005; 365:153–165.
Article2. Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008; 134:1570–1595.
Article3. Hotta T, Takifuji K, Arii K, Yokoyama S, Matsuda K, Higashiguchi T, et al. Potential predictors of long-term survival after surgery for patients with stage IV colorectal cancer. Anticancer Res. 2006; 26:1377–1383.4. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010; 97:1110–1118.
Article5. Mahmoud N, Bullard Dunn K. Metastasectomy for stage IV colorectal cancer. Dis Colon Rectum. 2010; 53:1080–1092.
Article6. Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010; 30:653–660.7. Stelzner S, Hellmich G, Koch R, Ludwig K. Factors predicting survival in stage IV colorectal carcinoma patients after palliative treatment: a multivariate analysis. J Surg Oncol. 2005; 89:211–217.
Article8. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.9. Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg. 2011; 35:684–692.
Article10. Chew MH, Teo JY, Kabir T, Koh PK, Eu KW, Tang CL. Stage IV colorectal cancers: an analysis of factors predicting outcome and survival in 728 cases. J Gastrointest Surg. 2012; 16:603–612.
Article11. de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multiinstitutional analysis of 1669 patients. Ann Surg. 2009; 250:440–448.12. D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011; 18:1096–1103.13. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008; 247:125–135.14. Nitsche U, Maak M, Künzli B, Schuster T, Friess H, Rosenberg R. Prognostic factors and survival improvements in stage IV colorectal cancer*. Eur Surg. 2012; 44:47–53.
Article15. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of tumor pathology for postoperative survival of patients undergoing surgery for stage IV colorectal cancer. Anticancer Res. 2012; 32:3291–3297.16. Law WL, Chan WF, Lee YM, Chu KW. Non-curative surgery for colorectal cancer: critical appraisal of outcomes. Int J Colorectal Dis. 2004; 19:197–202.
Article17. Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005; 12:900–909.
Article18. Katoh H, Yamashita K, Kokuba Y, Satoh T, Ozawa H, Hatate K, et al. Surgical resection of stage IV colorectal cancer and prognosis. World J Surg. 2008; 32:1130–1137.
Article19. Platell C, Ng S, O'bichere A, Tebbutt N. Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum. 2011; 54:214–219.
Article20. House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010; 210:744–752.
Article21. Gao P, Song YX, Wang ZN, Xu YY, Tong LL, Sun JX, et al. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2013; 13:123.
Article22. Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009; 15:6391–6397.
Article23. Park JH, Kim TY, Lee KH, Han SW, Oh DY, Im SA, et al. The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer. 2013; 108:1425–1431.
Article24. Karoui M, Roudot-Thoraval F, Mesli F, Mitry E, Aparicio T, Des Guetz G, et al. Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum. 2011; 54:930–938.
Article25. Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014; 21:3900–3908.
Article26. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
Article27. Haq AI, Schneeweiss J, Kalsi V, Arya M. The Dukes staging system: a cornerstone in the clinical management of colorectal cancer. Lancet Oncol. 2009; 10:1128.
Article28. Sobin LH. TNM: principles, history, and relation to other prognostic factors. Cancer. 2001; 91:8 Suppl. 1589–1592.29. Foster JH. History of liver surgery. Arch Surg. 1991; 126:381–387.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pattern of Recurrences and Metastases according to Regional Lymph Node Metastasis of Colorectal Cancer
- Prognostic Value of HSP 70 in Colorectal Cancer
- The Prognosis of Patients with Stage IV Gastric Carcinoma without Distant Metastasis
- Clinical Implications of Lymph Node Metastasis in Colorectal Cancer: Current Status and Future Perspectives
- Clinical Significance of Lymph Node Micrometastasis in Patients with Dukes' B Colorectal Cancer