J Korean Med Sci.
2004 Dec;19(6):879-886. 10.3346/jkms.2004.19.6.879.
Upregulation of VEGF and FGF2 in Normal Rat Brain after Experimental Intraoperative Radiation Therapy
- Affiliations
-
- 1Department of Neurosurgery, Medical College, Korea University, Seoul, Korea. yongku9@chollian.net
- 2Department of Radiation Oncology, Medical College, Korea University, Seoul, Korea.
- 3Department of Pathology, Medical College, Korea University, Seoul, Korea.
- KMID: 1778573
- DOI: http://doi.org/10.3346/jkms.2004.19.6.879
Abstract
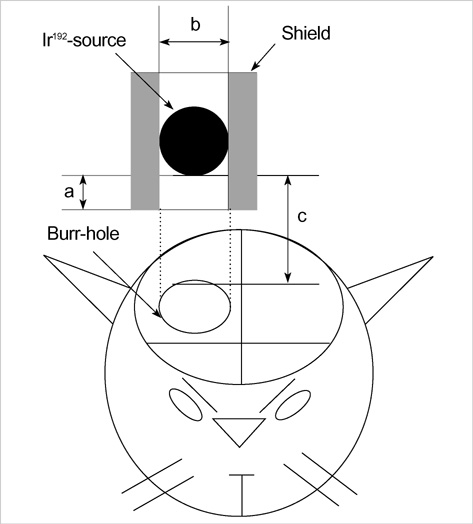
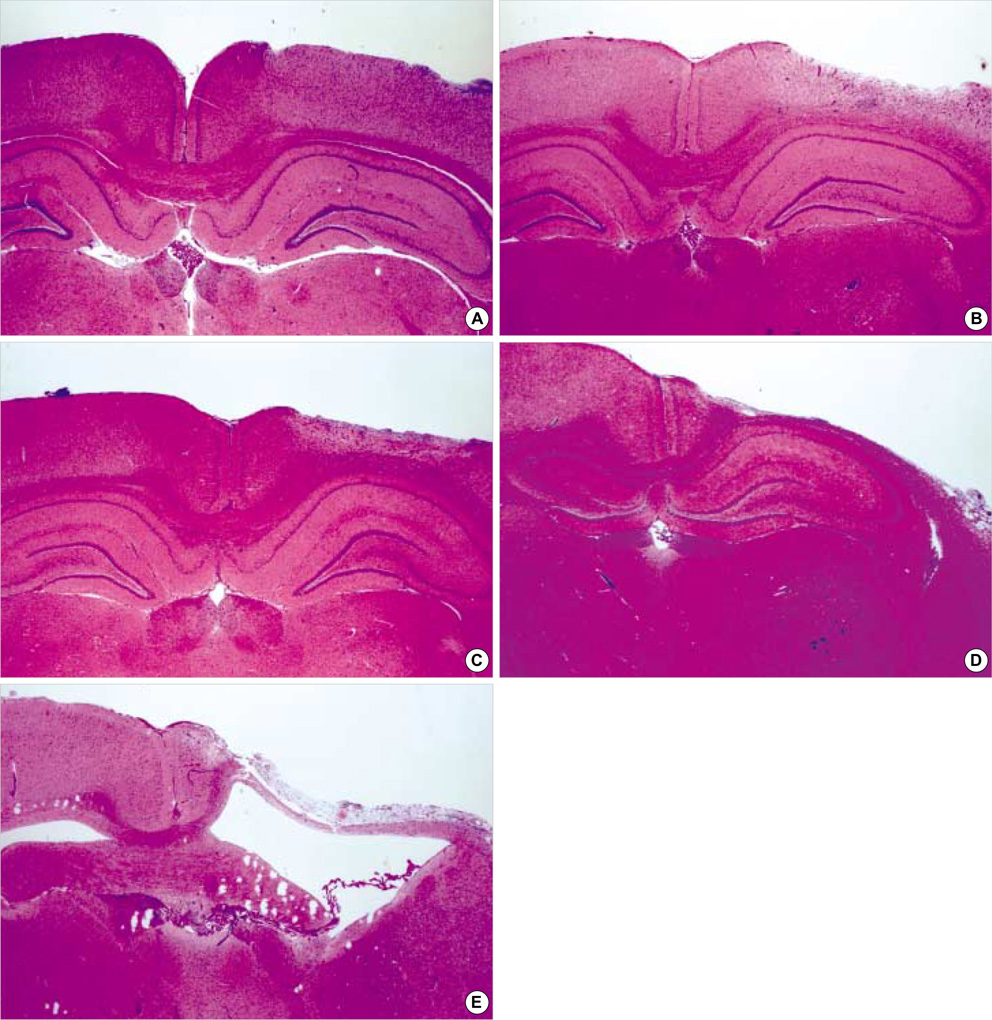
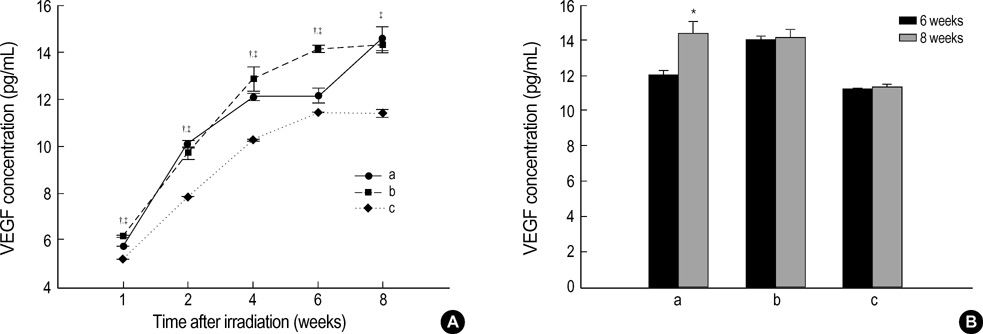
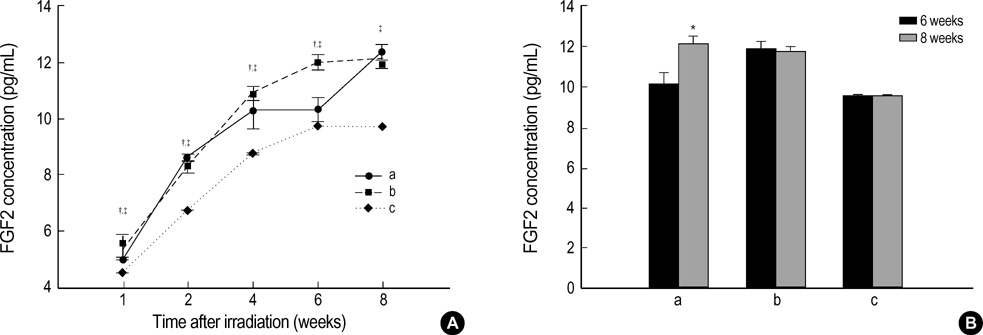
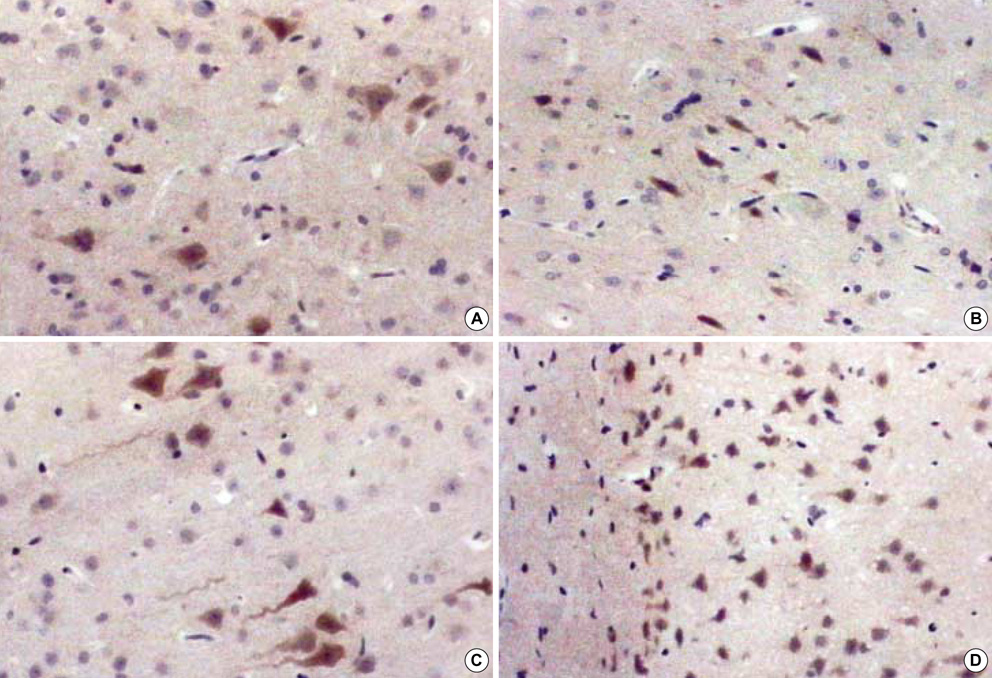
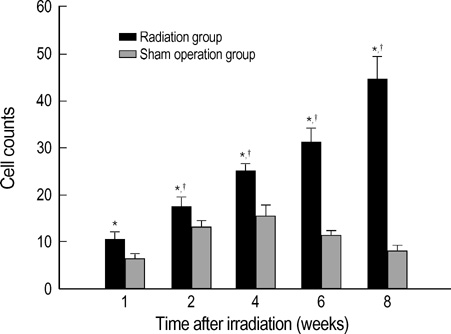
- The expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)2 in the irradiated brain was examined to test how a single high dose radiation, similar to that used for intraoperative radiation therapy given to the normal cerebrum, can affect the vascular endothelium. After a burr hole trephination in the rat skull, the cerebral hemisphere was exposed to a single 10 Gy dose of gamma rays, and the radiation effect was assessed at 1, 2, 4, 6, and 8 weeks after irradiation. His-tological changes, such as reactive gliosis, inflammation, vascular proliferation and necrosis, were correlated with the duration after irradiation. Significant VEGF and FGF2 expression in the 2- and 8-week were detected by enzyme-linked immunosorbent assay quantification in the radiation group. Immunohistochemical study for VEGF was done and the number of positive cells gradually increased over time, compared with the sham operation group. In conclusion, the radiation injuries consisted of radiation necrosis associated with the expression of VEGF and FGF2. These findings indicate that VEGF and FGF2 may play a role in the radiation injuries after intraoperative single high-dose irradiation.
Keyword
MeSH Terms
-
Animals
Brain/metabolism/pathology/radiation effects
Brain Injuries/etiology/*metabolism/*pathology
Fibroblast Growth Factor 2/*metabolism
Necrosis
Radiation Injuries/*pathology
Radiosurgery/*adverse effects
Rats
Rats, Sprague-Dawley
Up-Regulation/radiation effects
Vascular Endothelial Growth Factor A/*metabolism
Figure
Reference
-
1. Matsutani M, Nakamura O, Asai A. Intraoperative radiation therapy for glioblastoma multiforme. Saishin Igaku. 1986. 41:1506–1513.
Article2. Kim JH, Chung YG, Kim HK, Kim CY, Lee HK, Lee KC. Pathological changes in the rat brain after experimental intraoperative radiation. J Korean Neurosurg Soc. 1997. 26:1502–1512.3. Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989. 7:287–294.
Article4. Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000. 60:321–327.5. Zagzag D. Angiogenic growth factors in neural embryogenesis and neoplasia. Am J Pathol. 1995. 146:293–309.6. Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992. 59:115–165.
Article7. Finklestein SP, Apostolides PJ, Caday CG, Prosser J, Philips MF, Klagsbrun M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res. 1988. 460:253–259.
Article8. Janet T, Grothe C, Pettmann B, Unsicker K, Sensenbrenner M. Immunocytochemical demonstration of fibroblast growth factor in cultured chick and rat neurons. J Neurosci Res. 1988. 19:195–201.
Article9. Kniss DA, Burry RW. Serum and fibroblast growth factor stimulate quiescent astrocytes to re-enter the cell cycle. Brain Res. 1988. 439:281–288.
Article10. Moscatelli D, Rifkin DB. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988. 948:67–85.
Article11. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992. 359:845–848.
Article12. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983. 219:983–985.
Article13. Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993. 53:5822–5827.14. Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996. 271:2746–2753.
Article15. Chung YG, Kim CY, Lee HK, Lee KC, Chu JW, Choi MS. Preliminary experiences with intraoperative radiation therapy (IORT) for the treatment of brain tumors. J Korean Med Sci. 1995. 10:449–452.
Article16. Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988. 61:1043–1052.
Article17. Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992. 114:521–532.
Article18. Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischaemia in the rat. Neurosci Lett. 1998. 249:79–82.19. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its recepters. FASEB J. 1999. 13:9–22.20. Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994. 71:374–379.21. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992. 359:843–845.
Article22. Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999. 58:313–320.23. Bartholdi D, Rubin BP, Schwab ME. VEGF mRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur J Neurosci. 1997. 9:2549–2560.
Article24. Stewart PA, Vinters HV, Wong CS. Blood-spinal cord barrier function and morphometry after single doses of x-rays in rat spinal cord. Int J Radiat Oncol Biol Phys. 1995. 32:703–711.
Article25. Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 1997. 72:45–53.26. Vaquero J, Zurita M, Cincu R. Vascular endothelial growth-permeability factor in granulation tissue of chronic subdural haematomas. Acta Neurochir (Wien). 2002. 144:343–347.27. Shore PM, Jackson EK, Wisniewski SR, Clark RS, Adelson PD, Kochanek PM. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery. 2004. 54:605–612.
Article28. Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-β 1 in serum of patients with acute ischemic stroke. Stroke. 2000. 31:1863–1870.29. Hanneken A, Lutty GA, McLeod DS, Robey F, Harvey AK, Hjelmeland LM. Localization of basic fibroblast growth factor to the developing capillaries of the bovine retina. J Cell Physiol. 1989. 138:115–120.
Article30. Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988. 130:393–400.31. Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989. 28:1737–1743.
Article32. Ding I, Huang K, Wang X, Greig JR, Miller RW, Okunieff P. Radioprotection of hematopoietic tissue by fibroblast growth factors in fractionated radiation experiments. Acta Oncol. 1997. 36:337–340.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of EMMPRIN and VEGF in the Rat Brain after Gamma Irradiation
- Pathological Changes in the Rat Brain after Experimental Intraoperative Radiation
- Suppression of VEGF and STAT3 by Lipoic acid in Experimental Diabetic Rat Retina
- The Prognostic Effect of VEGF Expression in Squamous Cell Carcinoma of the Cervix Treated with Radiation Therapy Alone
- An Experimental Study on The of Hyperthermia on Radiation Therapy of Mammary Carcinoma of Rat