J Korean Med Sci.
2006 Jun;21(3):436-440. 10.3346/jkms.2006.21.3.436.
Improved Survival in Patients with Recurrent Wilms Tumor: the Experience of the Seoul National University Children's Hospital
- Affiliations
-
- 1Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju, Korea.
- 2Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hyshin@plaza.snu.ac.kr
- KMID: 1778424
- DOI: http://doi.org/10.3346/jkms.2006.21.3.436
Abstract
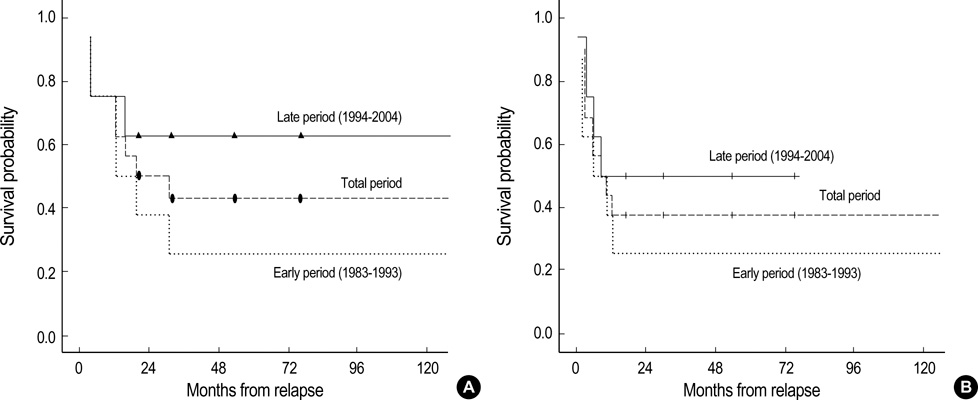
- The survival in cases with relapsed Wilms tumor is dismal. Recently, however the introduction of new therapeutic agents and experimental strategies has improved the survival. We analysed the survival of patients with relapsed Wilms tumor according to the treatment period. During the early period 1983-1993, patients who had received two drugs were treated with doxorubicin and the others were treated with cisplatin and etoposide, whereas during the late period 1994-2004, patients were treated with combinations of cyclophosphamide/etoposide and carboplatin/etoposide. During the early period, 8 of 57 experienced relapse, and 8 of 41 relapsed during the late period. Only 2 patients treated during the early period survived in complete response (CR), whereas during the late period, 5 patients remained alive in CR, and 3 of those received high-dose chemotherapy (HDC) with autologous peripheral stem cell rescue (SCR). The estimated 5 yr event-free survival rate was 37.5% in the entire study group, 50% for patients in the late period, and 25% for patients in the early period (p=0.38). The survival in patients with relapsed Wilms tumor dramatically improved during the late period and HDC with SCR was one of the effective salvage strategies.
Keyword
MeSH Terms
Figure
Reference
-
1. Grundy P, Breslow N, Green DM, Sharples K, Evans A, D'Angio GJ. Prognostic factors for children with recurrent Wilms' tumor: results from the Second and Third National Wilms' Tumor Study. J Clin Oncol. 1989. 7:638–647.
Article2. Pinkerton CR, Groot-Loonen JJ, Morris-Jones PH, Pritchard J. Response rates in relapsed Wilms tumor. A need for new effective agents. Cancer. 1991. 67:567–571.
Article3. Tournade MF, Lemerle J, Brunat-Mentigny M, Bachelot C, Roche H, Taboureau O, Olive D, Lejars O, Boilletot A, Demeocq F. Ifosfamide is an active drug in Wilms tumor: a phase II study conducted by the French Society of Pediatric Oncology. J Clin Oncol. 1988. 6:793–796.
Article4. Pein F, Pinkerton R, Tournade MF, Brunat-Mentigny M, Levitt G, Margueritte G, Rubie H, Sommelet D, Thyss A, Zucker JM. Etoposide in relapsed or refractory Wilms tumor: A phase II study by the French Society of Pediatric Oncology and the United Kingdom Children's Cancer Study Group. J Clin Oncol. 1993. 11:1478–1481.
Article5. Ettinger LJ, Gaynon PS, Krailo MD, Ru N, Baum ES, Siegel SE, Hammond GD. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Children's Cancer Group. Cancer. 1994. 15:1297–1301.
Article6. Pein F, Tournade MF, Zucker JM. Etoposide and carboplatin: a highly effective combination in relapsed or refractory Wilms' tumor-A phase II study by the French Society of Pediatric Oncology. J Clin Oncol. 1994. 12:931–936.
Article7. Kung FH, Pratt CB, Vega RA, Jaffe N, Strother D, Schwenn M, Nitschke R, Homans AC, Holbrook CT, Golembe B. Etoposide/Ifosfamide combination in the treatment of recurrent malignant solid tumors of childhood: A Pediatric Oncology Group Phase I/II study. Cancer. 1993. 71:1898–1903.8. Garaventa A, Hartmann O, Bernard JL, Zucker JM, Pardo N, Castel V, Dallorso S, Adelbost Z, Ladenstein R, Chauvin F, Phillip T. Autologous bone marrow transplantation for pediatric Wilms' tumor: the experience of the European Bone Marrow Transplantation Solid Tumor Registry. Med Pediatr Oncol. 1994. 22:11–14.
Article9. Pein F, Michon J, Valteau-Couanet D, Quintana E, Frappaz D, Vannier JP, Philip T, Bergeron C, Baranzelli MC, Thyss A, Stephan JL, Boutard P, Gentet JC, Zucker JM, Tournade MF, Hartmann O. High dose melphalan, etoposide, and carboplatin followed by autologous stem cell rescue in pediatric high risk recurrent Wilms' tumor: a French Society of Pediatric Oncology study. J Clin Oncol. 1998. 16:3295–3301.10. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, Hero B, Selle B, Niemeyer C, Finckenstein FG, Schulz A, Wawer A, Zintl F, Graf N. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002. 30:893–898.
Article11. Campbell AD, Cohn SL, Reynolds M, Seshadri R, Morgan E, Geissler G, Rademaker A, Marymount M, Kalapurakal J, Haut PR, Duerst R, Kletzel M. Treatment of relapsed Wilms tumor with high-dose therapy and autologous hematopoietic stem-cell rescue: the experience at Children's Memorial Hospital. J Clin Oncol. 2004. 15:2885–2890.
Article12. D'Angio GJ, Evans AE, Breslow N, Beckwith B, Bishop H, Feigl P, Goodwin W, Leape LL, Sinks LF, Sutow W, Tefft M, Wolff J. The treatment of Wilms' tumor: Result of National Wilms' Tumor Study. Cancer. 1976. 38:633–646.13. Tannous R, Giller R, Holmes E. Intensive therapy for high-risk relapsed Wilms' tumor. A CCG-4921/POG-9445 study report. Proc Am Soc Clin Oncol. 2000. 19:(Abstr 2315).14. Suh WS, Kang IJ, Koo HH, Kook H, Kim SK, Kim HK, Kim HM, Kim HS, Park KD, Park KB, Park SK, Park JS, Park JE, Park HJ, Seo JJ, Sung KW, Shin HY, Ahn HS, Yang CH, Yoo KH, Yoo ES, Lyu CJ, Lee KC, Lee KS, Lee SY, Lee YH, Lim YT, Jang PS, Chung NG, Jeong DC, Jung HL, Cho DW, Cho B, Choi YM, Hah JO, Hwang PH, Hwang TJ. Epidemiology and clinical outcomes of childhood Wilms tumor in Korea. Korea J Pediatr Hematol-Oncol. 2004. 11:164–170.15. Choi YD, Han SW, Choi SK, Ko WJ, Lee JS, Lee SJ, Han SJ, Hwang EH, Yu CJ, Kim BS. Prognostic factors and survival rates of preoperative and recurred cases chemotherapy in Wilms' tumor. Korean J Urol. 2000. 41:741–746.16. Kung FH, Desai SJ, Dickerman JD, Goorin AM, Harris MB, Inoue S, Krischer JP, Murphy SB, Pratt CB, Toledano S, Wiley JM, Yu AL. Ifosfamide, carboplatin, etoposide (ICE) for recurrent malignant solid tumors of childhood: a Pediatric Oncology Group Phase I/II study. J Pediatr Hematol Oncol. 1995. 17:265–269.17. Abu-Ghosh AM, Krailo MD, Glodman SC, Slack RS, Davenport V, Morris E, Laver JH, Reaman GH, Cairo MS. Ifosfamide, carboplatin and etoposide in children with poor-risk relapsed Wilms' tumor: a Children's Cancer Group report. Ann oncol. 2002. 13:460–469.
Article