J Korean Med Sci.
2010 Mar;25(3):458-465. 10.3346/jkms.2010.25.3.458.
Improved Outcome of Central Nervous System Germ Cell Tumors: Implications for the Role of Risk-adapted Intensive Chemotherapy
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. shinhj@skku.edu
- KMID: 1778044
- DOI: http://doi.org/10.3346/jkms.2010.25.3.458
Abstract
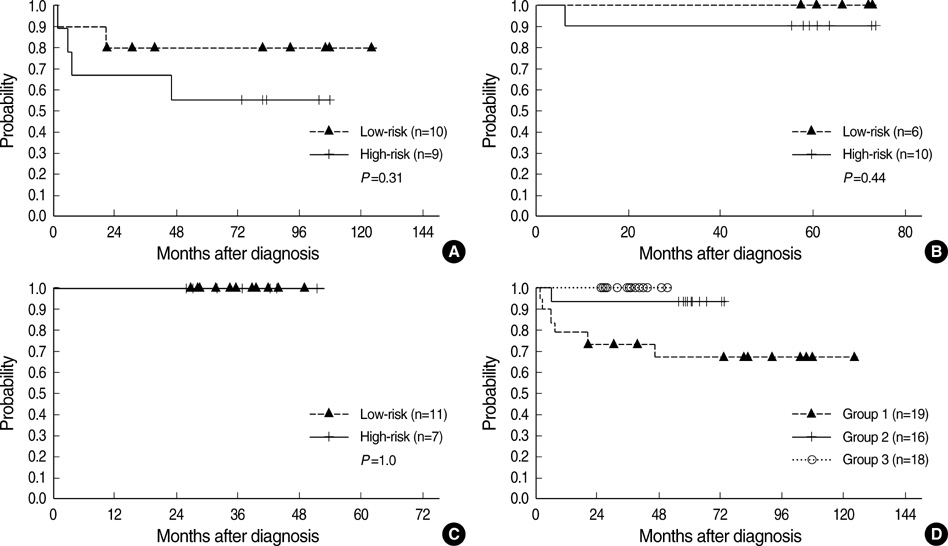
- To determine the impact of treatment protocols on the outcome of central nervous system germ cell tumors (CNS-GCTs), we reviewed the medical records of 53 patients who received front-line chemotherapy from September 1997 to September 2006. Pure germinoma, normal alpha-fetoprotein level and beta-human chorionic gonadotropin level <50 mIU/mL were regarded as low-risk features and the others as high-risk. Patients from different time periods were divided into 3 groups according to the chemotherapy protocols. Group 1 (n=19) received 4 cycles of chemotherapy comprising cisplatin, etoposide and bleomycin. Group 2 (n=16) and group 3 (n=18) received 4 cycles of chemotherapy with cisplatin, etoposide, cyclophosphamide and vincristine in the former and with carboplatin, etoposide, cyclophosphamide and bleomycin in the latter. In group 2 and group 3, high-risk patients received double doses of cisplatin, carboplatin and cyclophosphamide. Radiotherapy was given after chemotherapy according to the clinical requirements. The event-free survivals of groups 1, 2, and 3 were 67.0%, 93.8%, and 100%, respectively (group 1 vs. 2, P=0.06; group 2 vs. 3, P=0.29; group 1 vs. 3, P=0.02). Our data suggest that risk-adapted intensive chemotherapy may improve the outcome of patients with malignant CNS-GCTs.
MeSH Terms
-
Adolescent
Antineoplastic Agents/*therapeutic use
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Central Nervous System Neoplasms/pathology/*therapy
Child
Combined Modality Therapy
Disease-Free Survival
Female
Humans
Kaplan-Meier Estimate
Male
Neoplasms, Germ Cell and Embryonal/pathology/*therapy
*Radiotherapy
Risk Factors
Treatment Outcome
Tumor Markers, Biological/metabolism
Young Adult
Antineoplastic Agents
Tumor Markers, Biological
Figure
Reference
-
1. Cho KT, Wang KC, Kim SK, Shin SH, Chi JG, Cho BK. Pediatric brain tumors: statistics of SNUH, Korea (1959-2000). Childs Nerv Syst. 2002. 18:30–37.
Article2. Committee of Brain Tumor Registry of Japan. Report of Brain Tumor Registry of Japan (1969-1996). Neurol Med Chir (Tokyo). 2003. 43:Suppl. i–vii. 1–111.3. Wong TT, Ho DM, Chang KP, Yen SH, Guo WY, Chang FC, Liang ML, Pan HC, Chung WY. Primary pediatric brain tumors: statistics of Taipei VGH, Taiwan (1975-2004). Cancer. 2005. 104:2156–2167.4. Sung DI, Harisliadis L, Chang CH. Midline pineal tumors and suprasellar germinomas: highly curable by irradiation. Radiology. 1978. 128:745–751.
Article5. Rich TA, Cassady JR, Strand RD, Winston KR. Radiation therapy for pineal and suprasellar germ cell tumors. Cancer. 1985. 55:932–940.
Article6. Sakai N, Yamada H, Andoh T, Hirata T, Shimizu K, Shinoda J. Primary intracranial germ-cell tumors. A retrospective analysis with special reference to long-term results of treatment and the behavior of rare types of tumors. Acta Oncol. 1988. 27:43–50.
Article7. Shibamoto Y, Abe M, Yamashita J, Takahashi M, Hiraoka M, Ono K, Tsutsui K. Treatment results of intracranial geminoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys. 1988. 15:285–290.8. Sano K, Matsutani M, Seto T. So-called intracranial germ cell tumours: personal experiences and a theory of their pathogenesis. Neurol Res. 1989. 11:118–126.
Article9. Wolden SL, Wara WM, Larson DA, Prados MD, Edwards MS, Sneed PK. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys. 1995. 32:943–949.
Article10. Huh SJ, Shin KH, Kim IH, Ahn YC, Ha SW, Park CI. Radiotherapy of intracranial germinoma. Radiother Oncol. 1996. 38:19–23.11. Hardenbergh PH, Golden J, Billet A, Scott RM, Shrieve DC, Silver B, Loeffler JS, Tarbell NJ. Intracranial germinoma: the case for lower dose radiation therapy. Int J Radiat Oncol Biol Phys. 1997. 39:419–426.
Article12. Bamberg M, Kortmann RD, Calaminus G, Becker G, Meisner C, Harms D, Gobel U. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999. 17:2585–2592.
Article13. Kirshner JJ, Ginsberg SJ, Fitzpatrick AV, Comis RL. Treatment of a primary intracranial germ cell tumor with systemic chemotherapy. Med Pediatr Oncol. 1981. 9:361–365.
Article14. Allen JC, Bosl G, Walker R. Chemotherapy trials in recurrent primary intracranial germ cell tumors. J Neurooncol. 1985. 3:147–152.
Article15. Kobayashi T, Yoshida J, Ishiyama J, Noda S, Kito A, Kida Y. Combination chemotherapy with cisplatin and etoposide for malignant intracranial germ-cell tumors. An experimental and clinical study. J Neurosurg. 1989. 70:676–681.16. Packer RJ, Cohen BH, Coney K. Intracranial germ cell tumors. Oncologist. 2000. 5:312–320.
Article17. Itoyama Y, Kochi M, Kuratsu J, Takamura S, Kitano I, Marubayashi T, Uemura S, Ushio Y. Treatment of intracranial nongerminomatous malignant germ cell tumors producing alpha-fetoprotein. Neurosurgery. 1995. 36:459–464.18. Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca JG, Kellie S, Maher P, Vlamis V, Walker RW, Leibel S, Finlay JL. Chemotherapy without irradiation--a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996. 14:2908–2915.
Article19. Baranzelli MC, Patte C, Bouffet E, Portas M, Mechinaud-Lacroix F, Sariban E, Roche H, Kalifa C. An attempt to treat pediatric intracranial alphaFP and betaHCG secreting germ cell tumors with chemotherapy alone. SFOP experience with 18 cases. Societe Francaise d'Oncologie Pediatrique. J Neurooncol. 1998. 37:229–239.20. Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997. 32:71–80.21. Matsutani M, Ushio Y, Abe H, Yamashita J, Shibui S, Fujimaki T, Takakura K, Nomura K, Tanaka R, Fukui M, Yoshimoto T, Hayakawa T, Nagashima T, Kurisu K, Kayama T. Combined chemotherapy and radiation therapy for central nervous system germ cell tumors: preliminary results of a Phase II study of the Japanese Pediatric Brain Tumor Study Group. Neurosurg Focus. 1998. 5:e7.
Article22. Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, Shikama N, Saeki N, Yoshii Y, Murayama S. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003. 98:369–376.23. Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M. Pre-radiation chemotherapy with response-based radiation therapy in children with centeral nervous system germ cell tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2007. 48:285–291.24. Yasumasu T, Ueda T, Uozumi J, Mihara Y, Kumazawa J. Comparative study of cisplatin and carboplatin on pharmacokinetics, nephrotoxicity and effect on renal nuclear DNA synthesis in rats. Pharmacol Toxicol. 1992. 70:143–147.
Article25. De Lauretis A, De Capua B, Barbieri MT, Bellussi L, Passali D. ABR evaluation of ototoxicity in cancer patients receiving cisplatin or carboplatin. Scand Audiol. 1999. 28:139–143.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of the Ovarian Germ Cell Tumors
- Cytologic Features of Intracranial Germ Cell Tumors in Crush Preparation
- Understanding the Treatment Strategies of Intracranial Germ Cell Tumors: Focusing on Radiotherapy
- Recent advances in the management of primary central nervous system lymphoma
- Current Trends in Management for Central Nervous System Germ Cell Tumor