J Korean Med Sci.
2011 May;26(5):625-630. 10.3346/jkms.2011.26.5.625.
Plasma proGRP Concentration is Sensitive and Specific for Discriminating Small Cell Lung Cancer from Nonmalignant Conditions or Non-small Cell Lung Cancer
- Affiliations
-
- 1Brain Korea 21 Project, Center for Biomedical Human Resources, Chonnam National University, Gwangju, Korea.
- 2Department of Internal Medicine, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Laboratory Medicine, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea. mgshin@chonnam.ac.kr
- 4Abbott Diagnostics, Seoul, Korea.
- KMID: 1777860
- DOI: http://doi.org/10.3346/jkms.2011.26.5.625
Abstract
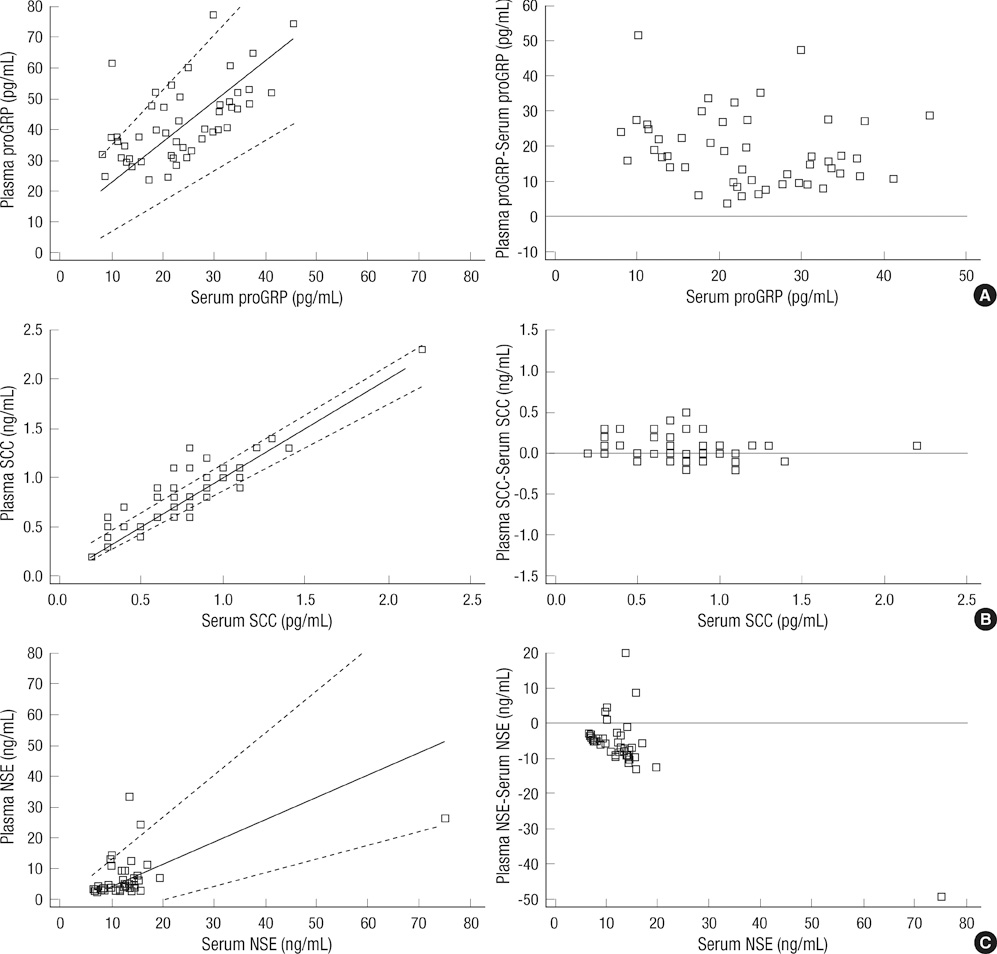
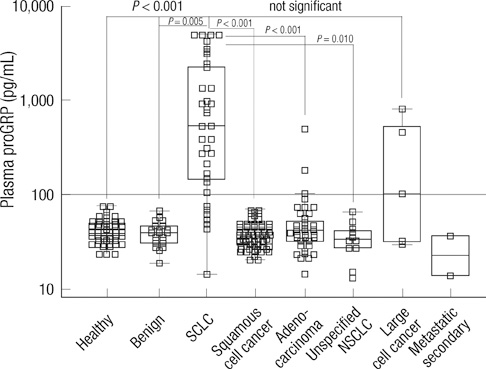
- To date, most clinical data on pro-gastrin-releasing peptide (proGRP) have been based on serum concentrations. This study evaluated the agreement between proGRP levels in fresh serum and plasma in patients with various lung diseases. Pairs of serum and EDTA plasma were collected from 49 healthy individuals. At the same time, EDTA plasma of 118 lung cancer patients and 23 patients with benign pulmonary diseases were prospectively collected. Compared to serum, plasma proGRP concentrations were higher by an average of 103.3%. Plasma proGRP was higher in malignancy (336.4 +/- 925.4 pg/mL) than in benign conditions (40.1 +/- 11.5 pg/mL). Small cell lung cancer (SCLC) patients showed higher levels of proGRP (1,256.3 +/- 1,605.6 pg/mL) compared to other types of lung cancer. Based on the ROC curve analyses at a specificity of 95%, the diagnostic sensitivity of plasma proGRP was estimated to be 83.8% in distinguishing SCLC from all the other conditions, and 86.5% for discriminating SCLC from the nonmalignant cases. Among the SCLC cases, limited stage disease had lower levels of plasma proGRP than extensive disease. When measuring circulating levels of proGRP, the use of plasma is preferred over serum. Plasma proGRP has a potential marker for discriminating SCLC from nonmalignant conditions or non-small cell lung cancer.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Carcinoma, Non-Small-Cell Lung/*blood/diagnosis/pathology
Diagnosis, Differential
Female
Humans
Lung Diseases/*blood
Lung Neoplasms/*blood/diagnosis/pathology
Male
Middle Aged
Peptide Fragments/*blood
Recombinant Proteins/blood
Sensitivity and Specificity
Small Cell Lung Carcinoma/*blood/diagnosis/pathology
Tumor Markers, Biological/*blood
Figure
Reference
-
1. Paik KH, Park YH, Ryoo BY, Yang SH, Lee JC, Kim CH, Ki SS, Kim JM, Park MJ, Ahn HJ, Choi W, Chung JH. Prognostic value of immunohistochemical staining of p53, bcl-2, and Ki-67 in small cell lung cancer. J Korean Med Sci. 2006. 21:35–39.2. Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide (31-98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res. 1994. 54:2136–2140.3. Yamaguchi K, Abe K, Kameya T, Adachi I, Taguchi S, Otsubo K, Yanaihara N. Production and molecular size heterogeneity of immunoreactive gastrin-releasing peptide in fetal and adult lungs and primary lung tumors. Cancer Res. 1983. 43:3932–3939.4. Takada M, Kusunoki Y, Masuda N, Matui K, Yana T, Ushijima S, Iida K, Tamura K, Komiya T, Kawase I, Kikui N, Morino H, Fukuoka M. Pro-gastrin-releasing peptide (31-98) as a tumour marker of small-cell lung cancer: comparative evaluation with neuron-specific enolase. Br J Cancer. 1996. 73:1227–1232.5. Molina R, Filella X, Augé JM. ProGRP: a new biomarker for small cell lung cancer. Clin Biochem. 2004. 37:505–511.6. Stieber P, Dienemann H, Schalhorn A, Schmitt UM, Reinmiedl J, Hofmann K, Yamaguchi K. Pro-gastrin-releasing peptide (ProGRP)--a useful marker in small cell lung carcinomas. Anticancer Res. 1999. 19:2673–2678.7. Molina R, Auge JM, Alicarte J, Filella X, Viñolas N, Ballesta AM. Pro-gastrin-releasing peptide in patients with benign and malignant diseases. Tumour Biol. 2004. 25:56–61.8. Niho S, Nishiwaki Y, Goto K, Ohmatsu H, Matsumoto T, Hojo F, Ohe Y, Kakinuma R, Kodama T. Significance of serum pro-gastrin-releasing peptide as a predictor of relapse of small cell lung cancer: comparative evaluation with neuron-specific enolase and carcinoembryonic antigen. Lung Cancer. 2000. 27:159–167.9. Yamaguchi K, Aoyagi K, Urakami K, Fukutani T, Maki N, Yamamoto S, Otsubo K, Miyake Y, Kodama T. Enzyme-linked immunosorbent assay of pro-gastrin-releasing peptide for small cell lung cancer patients in comparison with neuron-specific enolase measurement. Jpn J Cancer Res. 1995. 86:698–705.10. Sunaga N, Tsuchiya S, Minato K, Watanabe S, Fueki N, Hoshino H, Makimoto T, Ishihara S, Saito R, Mori M. Serum pro-gastrin-releasing peptide is a useful marker for treatment monitoring and survival in small-cell lung cancer. Oncology. 1999. 57:143–148.11. Okusaka T, Eguchi K, Kasai T, Kurata T, Yamamoto N, Ohe Y, Tamura T, Shinkai T, Saijo N. Serum levels of pro-gastrin-releasing peptide for follow-up of patients with small cell lung cancer. Clin Cancer Res. 1997. 3:123–127.12. Kuwabara M, Shibata N, Ariyoshi Y. Lung cancer: progress in diagnosis and treatment. I. Diagnosis and physiopathology: 4. Progress in the study of tumor markers. Nippon Naika Gakkai Zasshi. 1997. 86:20–26.13. Banfi G, Parma P, Pontillo M. Stability of tumor markers CA 19.9, CA 125, and CA 15.3 in serum obtained from plain tubes and tubes containing thixotropic gel separator. Clin Chem. 1997. 43:2430–2431.14. Yoshimura T, Fujita K, Kawakami S, Takeda K, Chan S, Beligere G, Dowell B. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol. 2008. 29:224–230.15. Nordlund MS, Bjerner J, Warren DJ, Nustad K, Paus E. Progastrin-releasing peptide: stability in plasma/serum and upper reference limit. Tumour Biol. 2008. 29:204–210.16. Aoyagi K, Miyake Y, Urakami K, Kashiwakuma T, Hasegawa A, Kodama T, Yamaguchi K. Enzyme immunoassay of immunoreactive progastrin-releasing peptide (31-98) as tumor marker for small-cell lung carcinoma: development and evaluation. Clin Chem. 1995. 41:537–543.17. Gruber C, Hatz R, Reinmiedl J, Nagel D, Stieber P. CEA, cyfra 21-1, NSE, and proGRP in the diagnosis of lung cancer: a multivariate approach. J Lab Med. 2008. 32:361–371.18. Nisman B, Biran H, Ramu N, Heching N, Barak V, Peretz T. The diagnostic and prognostic value of ProGRP in lung cancer. Anticancer Res. 2009. 29:4827–4832.