J Korean Med Sci.
2011 Aug;26(8):1103-1107. 10.3346/jkms.2011.26.8.1103.
Fulminant Epstein-Barr Virus-associated T-cell Lymphoproliferative Disorder in an Immunocompetent Middle-aged Man Presenting with Chronic Diarrhea and Gastrointestinal Bleeding
- Affiliations
-
- 1Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea. meeyon@yonsei.ac.kr
- 2Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1777852
- DOI: http://doi.org/10.3346/jkms.2011.26.8.1103
Abstract
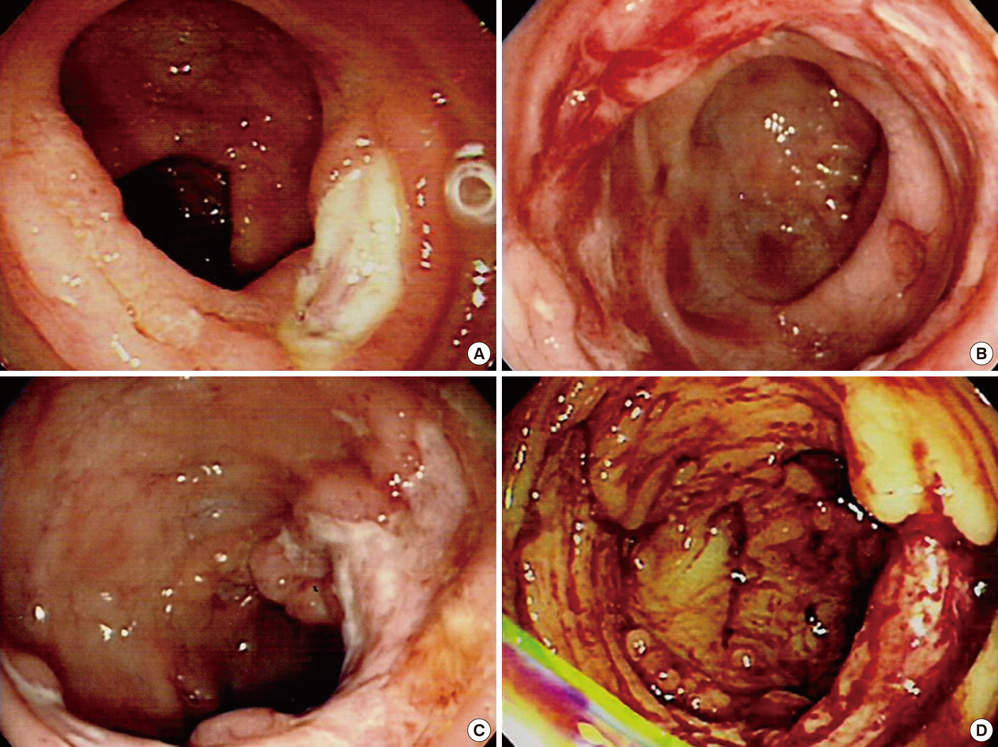
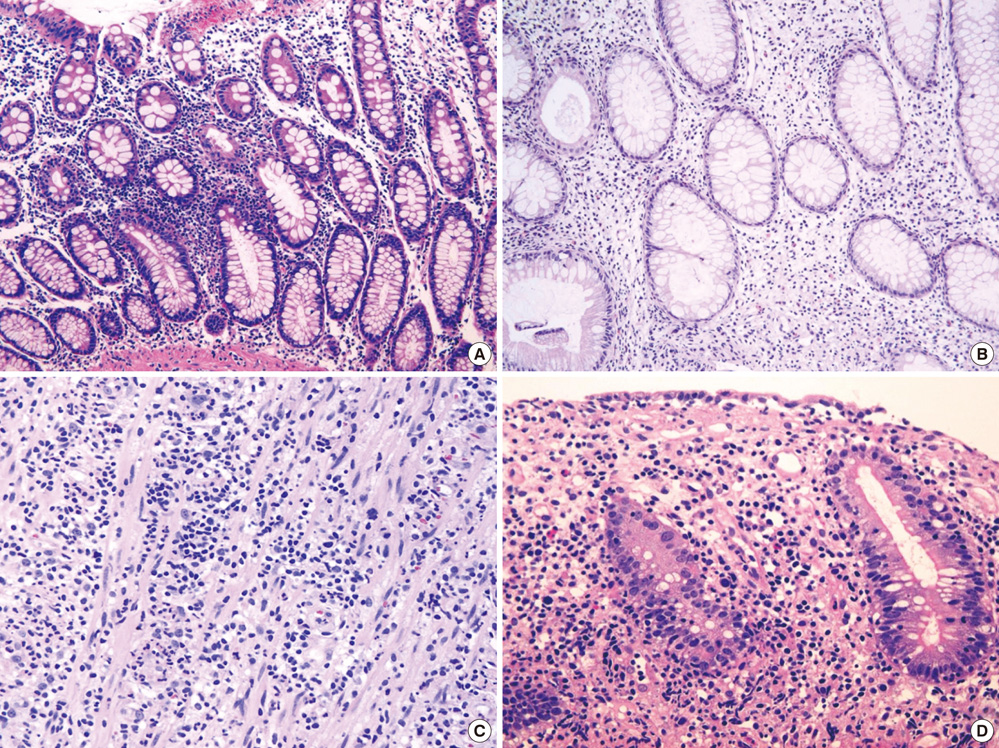
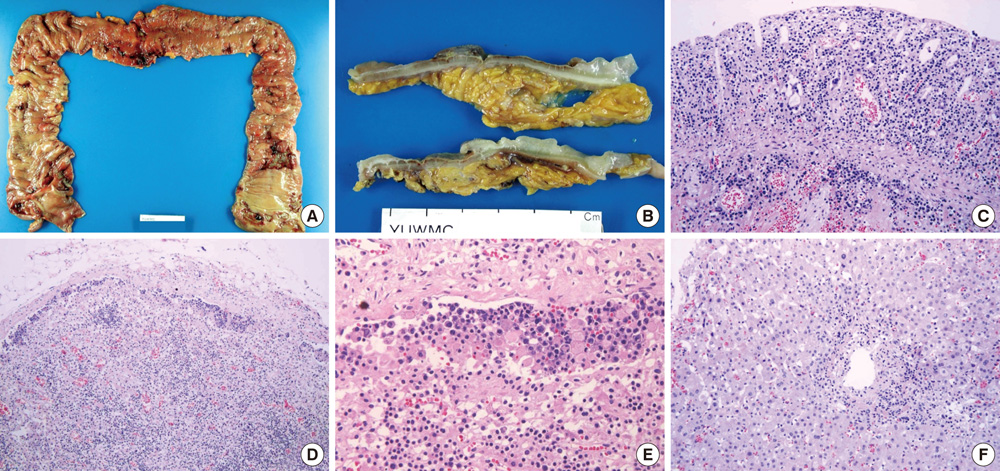
- The World Health Organization (WHO) recently defined systemic Epstein-Barr virus (EBV)-positive T-cell lymphoproliferative disorders (LPD) of childhood as a life-threatening illness. However, this rare disease has not been extensively studied. Here we report a case of systemic EBV-positive T-cell LPD in a previously healthy middle-aged man with a chief complaint of chronic diarrhea. The initial colon biopsy showed focal infiltration of EBV-positive small lymphocytes without any atypia. However, the disease rapidly progressed and the patient required a total colectomy due to severe gastrointestinal bleeding. Three and half months after admission, the patient died from a complication of disseminated intravascular coagulation. The resected colon showed diffuse infiltration of EBV-positive atypical lymphocytes with ischemic change. Most atypical lymphocytes were CD3+ or CD5+. The monoclonality of EBV was demonstrated by sequence variation analysis of the latent membrane protein 1 (LMP1) gene in the colectomy specimen as well as in the initial biopsy.
MeSH Terms
-
Chronic Disease
Colonoscopy
Diarrhea/*diagnosis
Disseminated Intravascular Coagulation/diagnosis
Epstein-Barr Virus Infections/complications/virology
Feces/virology
Gastrointestinal Hemorrhage
Herpesvirus 4, Human/genetics/*isolation & purification
Humans
Lymphoproliferative Disorders/*diagnosis/immunology/virology
Male
Middle Aged
RNA, Viral/analysis
T-Lymphocytes/*immunology/pathology
Figure
Reference
-
1. Hatachi S, Kunitomi A, Aozasa K, Yagita M. CD8(+) T-cell lymphoproliferative disorder associated with Epstein-Barr virus in a patient with rheumatoid arthritis during methotrexate therapy. Mod Rheumatol. 2010. 20:500–505.2. Kim MJ, Yun SH, Chun HK, Lee WY, Cho YB. Post-transplant lymphoproliferative disorder localized in the colon after liver transplantation: report of a case. Surg Today. 2009. 39:1076–1079.3. Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000. 343:481–492.4. Hauptmann S, Meru N, Schewe C, Jung A, Hiepe F, Burmester G, Niedobitek G, Buttgereit F. Fatal atypical T-cell proliferation associated with Epstein-Barr virus infection. Br J Haematol. 2001. 112:377–380.5. Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, Liao YL, Lin SH, Hsieh YC, Lu CL, Sheu MJ, Liu H. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009. 33:1230–1240.6. Quintanilla-Martinez L, Jaffe ES. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, Thiele J, Vardiman JW, editors. EBV-positiv T-cell lymphoproliferative disorders of childhood. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. Lyon: IARC.7. Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000. 96:443–451.8. Purtilo DT, Strobach RS, Okano M, Davis JR. Epstein-Barr virus-associated lymphoproliferative disorders. Lab Invest. 1992. 67:5–23.9. Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, Imai S, Okano M, Morio T, Yokota S, Tsuchiya S, Yachie A, Imashuku S, Kawa K, Wakiguchi H. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003. 187:527–533.10. Dolezal MV, Kamel OW, van de Rijn M, Cleary ML, Sibley RK, Warnke RA. Virus-associated hemophagocytic syndrome characterized by clonal Epstein-Barr virus genome. Am J Clin Pathol. 1995. 103:189–194.11. Craig FE, Clare CN, Sklar JL, Banks PM. T-cell lymphoma and the virus-associated hemophagocytic syndrome. Am J Clin Pathol. 1992. 97:189–194.12. Chan LC, Srivastava G, Pittaluga S, Kwong YL, Liu HW, Yuen HL. Detection of clonal Epstein-Barr virus in malignant proliferation of peripheral blood CD3+ CD8+ T cells. Leukemia. 1992. 6:952–956.13. Tashiro Y, Goto M, Takemoto Y, Sato E, Shirahama H, Utsunomiya A, Eizuru Y, Yonezawa S. Epstein-Barr virus-associated enteritis with multiple ulcers after stem cell transplantation: first histologically confirmed case. Pathol Int. 2006. 56:530–537.14. Pondarré C, Kebaili K, Dijoud F, Basset T, Philippe N, Bertrand Y. Epstein-Barr virus-related lymphoproliferative disease complicating childhood acute lymphoblastic leukemia: no recurrence after unrelated donor bone marrow transplantation. Bone Marrow Transplant. 2001. 27:93–95.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Adult Case of Severe Chronic Active Epstein-Barr Virus Infection with T-Cell Lymphoproliferative Disorder
- A Case of Severe Chronic Active Epstein-Barr Virus Infection with T-cell lymphoproliferative Disorder
- A Case of Epstein-Barr Virus-positive Diffuse, Large B-cell Lymphoma after Angioimmunoblastic T-cell Lymphoma
- Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorders
- CD30-Positive T-Cell Lymphoproliferative Disease of the Oral Mucosa in Children: A Manifestation of Epstein-Barr Virus-Associated T-Lymphoproliferative Disorder