Yonsei Med J.
2011 May;52(3):401-412. 10.3349/ymj.2011.52.3.401.
Neuron-Like Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea. mdkang@yonsei.ac.kr
- 2Department of Hemato-Oncology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Otorhinolaryngology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4FCB-Pharmicell Co., Ltd. Seongnam, Korea.
- KMID: 1777011
- DOI: http://doi.org/10.3349/ymj.2011.52.3.401
Abstract
- PURPOSE
Mesenchymal stem cells (MSCs) are multipotent and give rise to distinctly differentiated cells from all three germ layers. Neuronal differentiation of MSC has great potential for cellular therapy. We examined whether the cluster of mechanically made, not neurosphere, could be differentiated into neuron-like cells by growth factors, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).
MATERIALS AND METHODS
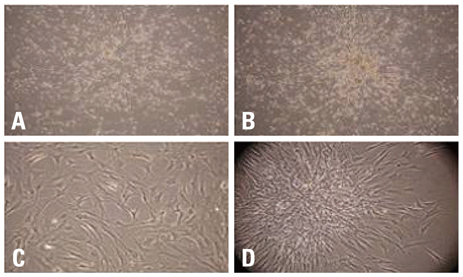
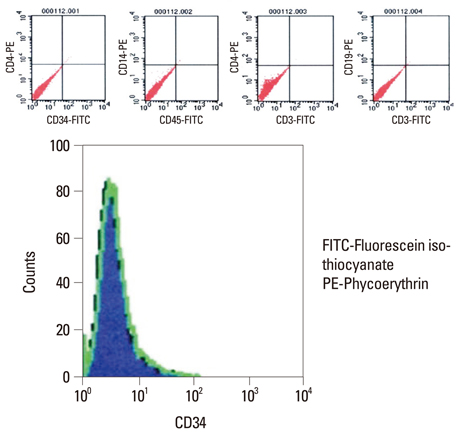
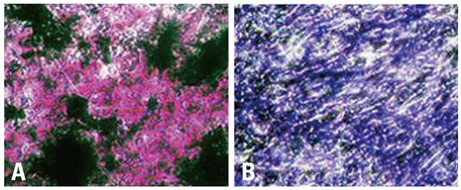
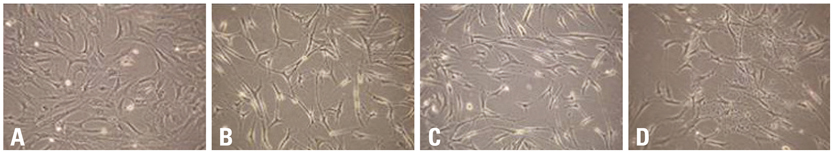
BMSCs grown confluent were mechanically separated with cell scrapers and masses of separated cells were cultured to form cluster BMSCs. As described here cluster of BMSCs were differentiated into neuron-like cells by EGF, HGF, and VEGF. Differentiated cells were analyzed by means of phase-contrast inverted microscopy, reverse transcriptase-polymerase chain reaction (RT-PCR), immunofluorescence, and immunocytochemistry to identify the expression of neural specific markers.
RESULTS
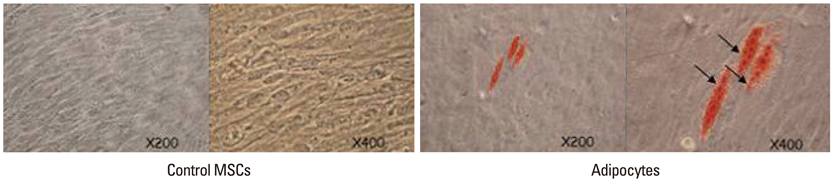
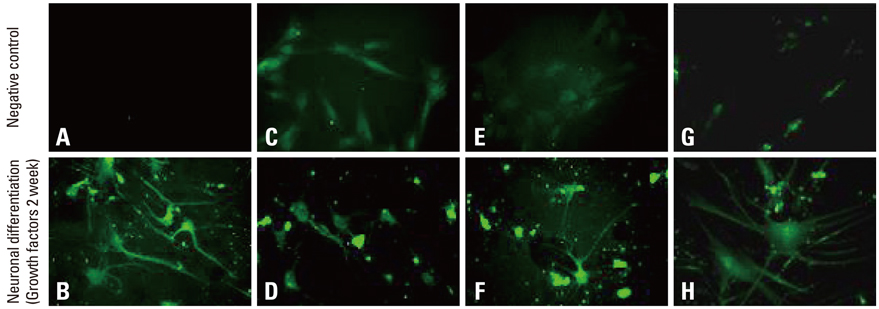
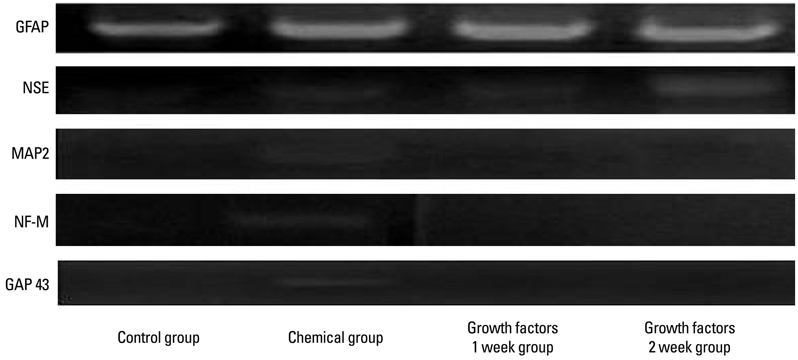
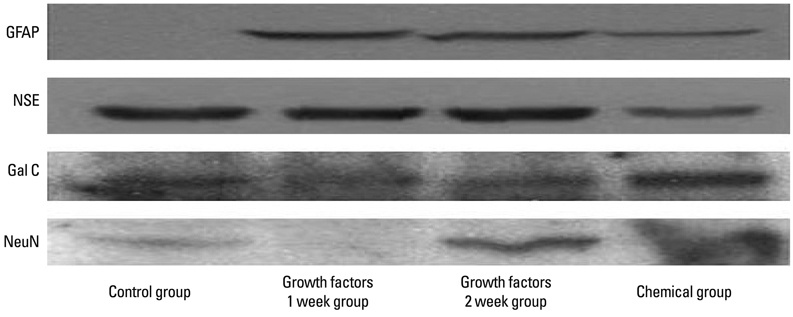
For the group with growth factors, the shapes of neuron-like cells was observable a week later, and two weeks later, most cells were similar in shape to neuron-like cells. Particularly, in the group with chemical addition, various shapes of filament structures were seen among the cells. These culture conditions induced MSCs to exhibit a neural cell phenotype, expressing several neuro-glial specific markers.
CONCLUSION
bone marrow-derived mesenchymal stem cells (BMSCs) could be easily induced to form clusters using mechanical scraping, not neurospheres, which in turn could differentiate further into neuron-like cells and might open an attractive possibility for clinical cell therapy for neurodegenerative diseases. In the future, we consider that neuron-like cells differentiated from clusters of BMSCs are needed to be compared and analyzed on a physiological and molecular biological level with preexisting neuronal cells, and studies on the possibility of their transplantation and differentiation capability in animal models are further required.
Keyword
MeSH Terms
-
Adult
Blotting, Western
Bone Marrow Cells/cytology/*drug effects
*Cell Culture Techniques
*Cell Differentiation
Cells, Cultured
Epidermal Growth Factor/pharmacology
Hepatocyte Growth Factor/pharmacology
Humans
Immunohistochemistry
Mesenchymal Stem Cells/cytology/*drug effects
Neurons/*cytology
Reverse Transcriptase Polymerase Chain Reaction
Vascular Endothelial Growth Factor A/pharmacology
Figure
Cited by 1 articles
-
Neurorestoration Induced by Mesenchymal Stem Cells: Potential Therapeutic Mechanisms for Clinical Trials
Jung Hwa Seo, Sung-Rae Cho
Yonsei Med J. 2012;53(6):1059-1067. doi: 10.3349/ymj.2012.53.6.1059.
Reference
-
1. Owen M. Peck WA, editor. Lineage of osteogenic cells and their relationship to the stromal system. Bone and mineral research. 1985. Amsterdam: Elsevier;1–25.2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article3. Tremain N, Korkko J, Ibberson D, Kopen GC, DiGirolamo C, Phinney DG. MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001. 19:408–418.
Article4. Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005. 7:36–45.
Article5. Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004. 14:311–324.
Article6. Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005. 102:186–191.
Article7. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000. 290:1775–1779.
Article8. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000. 290:1779–1782.
Article9. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article10. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005. 23:412–423.
Article11. Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004. 22:1263–1278.
Article12. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004. 22:1338–1345.13. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001. 98:2396–2402.
Article14. Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997. 97:561–570.
Article15. van den Bos C, Mosca JD, Winkles J, Kerrigan L, Burgess WH, Marshak DR. Human mesenchymal stem cells respond to fibroblast growth factors. Hum Cell. 1997. 10:45–50.16. Morse WR. Chinese Medicine. 1938. New York: Hoeber.17. Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997. 94:4080–4085.
Article18. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998. 95:3908–3913.
Article19. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999. 96:10711–10716.
Article20. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000. 164:247–256.
Article21. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article22. Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992. 12:4565–4574.
Article23. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992. 255:1707–1710.
Article24. Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996. 175:1–13.
Article25. Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992. 89:8591–8595.
Article26. Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999. 158:265–278.
Article27. Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989. 342:440–443.
Article28. Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997. 239:639–644.
Article29. Gage FH. Mammalian neural stem cells. Science. 2000. 287:1433–1438.
Article30. Björklund A, Lindvall O. Self-repair in the brain. Nature. 2000. 405:892–893. 895
Article31. Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002. 22:614–618.
Article32. Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999. 9:135–141.
Article33. Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995. 168:342–357.
Article34. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001. 344:710–719.
Article35. Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990. 247:574–577.
Article36. McKay R. Stem cells in the central nervous system. Science. 1997. 276:66–71.
Article37. Hüttmann A, Li CL, Dührsen U. Bone marrow-derived stem cells and "plasticity". Ann Hematol. 2003. 82:599–604.
Article38. Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003. 53:Suppl 3. S135–S146.
Article39. Silani V, Cova L, Corbo M, Ciammola A, Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004. 364:200–202.
Article40. Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003. 302:113–117.
Article41. Holden C, Vogel G. Stem cells. Plasticity: time for a reappraisal? Science. 2002. 296:2126–2129.42. Mejia-Aranguré JM, Fajardo-Gutiérrez A, Flores-Aguilar H, Martinez-García MC, Salamanca-Gómez F, Palma-Padilla V, et al. Environmental factors contributing to the development of childhood leukemia in children with Downs syndrome. Leukemia. 2003. 17:1905–1907.
Article43. Svendsen CN, Langston JW. Stem cells for Parkinson disease and ALS: replacement or protection? Nat Med. 2004. 10:224–225.
Article44. Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005. 193:312–325.
Article45. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
Article46. Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004. 303:1198–1201.
Article47. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.48. Noji S, Tashiro K, Koyama E, Nohno T, Ohyama K, Taniguchi S, et al. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990. 173:42–47.
Article49. Defrances MC, Wolf HK, Michalopoulos GK, Zarnegar R. The presence of hepatocyte growth factor in the developing rat. Development. 1992. 116:387–395.
Article50. Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993. 123:223–235.
Article51. Sato T, Hakeda Y, Yamaguchi Y, Mano H, Tezuka K, Matsumoto K, et al. Hepatocyte growth factor is involved in formation of osteoclast-like cells mediated by clonal stromal cells (MC3T3-G2/PA6). J Cell Physiol. 1995. 164:197–204.
Article52. Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ, et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U S A. 1996. 93:7644–7648.
Article53. Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995. 165:307–312.
Article54. Jung W, Castren E, Odenthal M, Vande Woude GF, Ishii T, Dienes HP, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. J Cell Biol. 1994. 126:485–494.
Article55. Honda S, Kagoshima M, Wanaka A, Tohyama M, Matsumoto K, Nakamura T. Localization and functional coupling of HGF and c-Met/HGF receptor in rat brain: implication as neurotrophic factor. Brain Res Mol Brain Res. 1995. 32:197–210.
Article56. Yamagata T, Muroya K, Mukasa T, Igarashi H, Momoi M, Tsukahara T, et al. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem Biophys Res Commun. 1995. 210:231–237.
Article57. Hamanoue M, Takemoto N, Matsumoto K, Nakamura T, Nakajima K, Kohsaka S. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J Neurosci Res. 1996. 43:554–564.
Article58. Ebens A, Brose K, Leonardo ED, Hanson MG Jr, Bladt F, Birchmeier C, et al. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996. 17:1157–1172.
Article59. Davey F, Hilton M, Davies AM. Cooperation between HGF and CNTF in promoting the survival and growth of sensory and parasympathetic neurons. Mol Cell Neurosci. 2000. 15:79–87.
Article60. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000. 97:12846–12851.
Article61. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002. 174:11–20.
Article62. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003. 73:778–786.
Article63. Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001. 189:49–57.
Article64. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002. 59:514–523.
Article65. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57:874–882.
Article66. Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007. 2:e1272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone marrow-derived stem cells contribute to regeneration of the endometrium