Yonsei Med J.
2013 Jan;54(1):1-14. 10.3349/ymj.2013.54.1.1.
The Role of Pharmacoethnicity in the Development of Cytotoxic and Molecular Targeted Drugs in Oncology
- Affiliations
-
- 1Japanese Society of Medical Oncology, Tokyo, Japan. saijo@jsmo.or.jp
- KMID: 1776911
- DOI: http://doi.org/10.3349/ymj.2013.54.1.1
Abstract
- The effective and toxic ranges of anticancer drugs are very narrow and, in some cases, inverted. Thus determination of the most appropriate dosage and schedule of administration is crucial for optimal chemotherapy. In common arm trials conducted in Japan and by Southwest Oncology Group (SWOG) that used the same doses and schedules for the administration of carboplatin plus paclitaxel, the frequency of hematological toxicity was significantly higher in the Japanese trials than in the SWOG trial, despite demonstrating similar response rates. The frequency of epidermal growth factor receptor (EGFR) mutations in tumors was significantly higher among East Asian populations, and these populations are also reported to demonstrate a higher response rates to epidermal growth factor receptor tyrosine-kinase inhibitors (EGFR-TKIs). The prevalence of interstitial lung disease induced by treatment with EGFR-TKIs has been shown to be quite high in the Japanese population. Clinical trials of cetuximab against non-small cell lung cancer and of bevacizumab against stomach cancer have shown that these agents are only active in Caucasians. In a trial examining the use of sorafenib after transarterial chemoembolization in Korean and Japanese patients with advanced hepatocellular carcinoma, the compliance and dose intensity of the drug were quite low compared with other trials. Although not only identified pharmacogenomics differences but also differences in social environment, and regional medical care, including pharmacoeconomics strongly influence ethnic differences in treatment response, further identification and understanding of the pharmacogenomics underlying ethnic differences will be essential to timely and reliable global development of new anticancer drugs.
Keyword
MeSH Terms
-
Antineoplastic Combined Chemotherapy Protocols/adverse effects/*therapeutic use
Asian Continental Ancestry Group
Carcinoma, Non-Small-Cell Lung/drug therapy/ethnology
Chemoembolization, Therapeutic
Clinical Trials as Topic
Drug Design
Ethnic Groups
Humans
Japan
Lung Diseases, Interstitial/chemically induced
Lung Neoplasms/drug therapy/ethnology
Mutation
Pharmacogenetics/*methods
Receptor, Epidermal Growth Factor/genetics
Republic of Korea
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009. 15:4806–4814.2. Huang RS, Ratain MJ. Pharmacogenetics and pharmacogenomics of anticancer agents. CA Cancer J Clin. 2009. 59:42–55.
Article3. Huang SM, Temple R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther. 2008. 84:287–294.
Article4. Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol. 2008. 26:2131–2138.
Article5. Lecomte T, Ferraz JM, Zinzindohoué F, Loriot MA, Tregouet DA, Landi B, et al. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004. 10:5880–5888.
Article6. Marsh S, Collie-Duguid ES, Li T, Liu X, McLeod HL. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999. 58:310–312.
Article7. Lal S, Wong ZW, Jada SR, Xiang X, Chen Shu X, Ang PC, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics. 2007. 8:567–575.
Article8. Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005. 23:6117–6125.
Article9. Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatr Blood Cancer. 2008. 50:769–771.
Article10. Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics. 2007. 17:497–504.
Article11. Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009. 27:2604–2614.
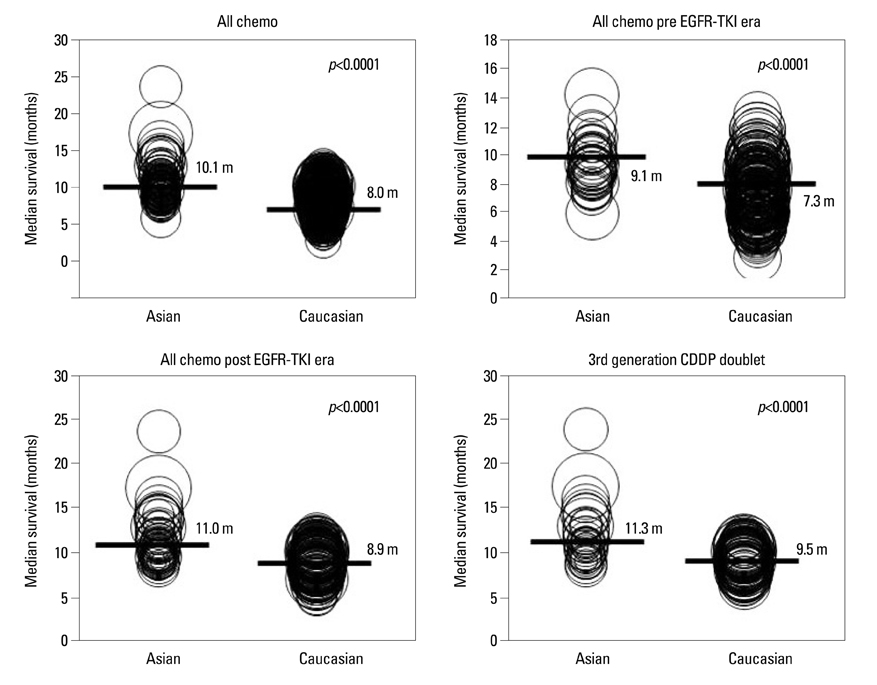
Article12. Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011. 6:1030–1038.
Article13. Toyooka S, Kiura K, Mitsudomi T. EGFR mutation and response of lung cancer to gefitinib. N Engl J Med. 2005. 352:2136.14. Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009. 27:3540–3546.
Article15. Lara PN Jr, Chansky K, Shibata T, Fukuda H, Tamura T, Crowley J, et al. Common arm comparative outcomes analysis of phase 3 trials of cisplatin + irinotecan versus cisplatin + etoposide in extensive stage small cell lung cancer: final patient-level results from Japan Clinical Oncology Group 9511 and Southwest Oncology Group 0124. Cancer. 2010. 116:5710–5715.
Article16. Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007. 18:317–323.
Article17. Kubota K, Kawahara M, Ogawara M, Nishiwaki Y, Komuta K, Minato K, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol. 2008. 9:1135–1142.
Article18. Williamson SK, Crowley JJ, Lara PN Jr, McCoy J, Lau DH, Tucker RW, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005. 23:9097–9104.
Article19. Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002. 346:85–91.
Article20. Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009. 27:2530–2535.
Article21. Nakagawa K, Tamura T, Negoro S, Kudoh S, Yamamoto N, Yamamoto N, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol. 2003. 14:922–930.
Article22. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002. 20:4292–4302.
Article23. Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002. 20:3815–3825.
Article24. Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002. 20:2240–2250.
Article25. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003. 21:2237–2246.
Article26. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.
Article27. Takahashi T, Yamamoto N, Nukiwa T, Mori K, Tsuboi M, Horai T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010. 30:557–563.28. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.
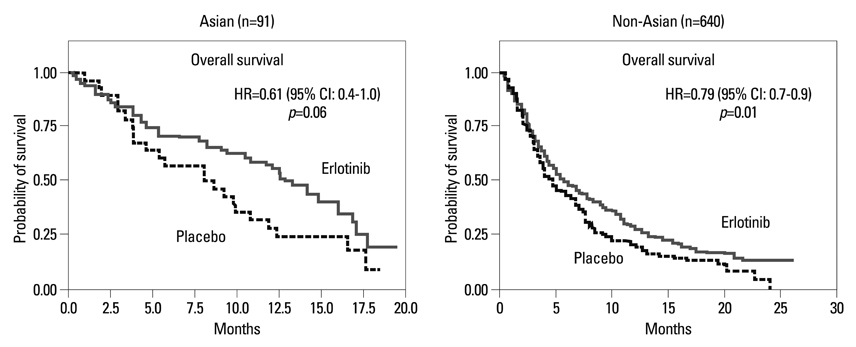
Article29. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.
Article30. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004. 22:777–784.
Article31. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004. 22:785–794.32. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005. 23:5892–5899.
Article33. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007. 25:1545–1552.
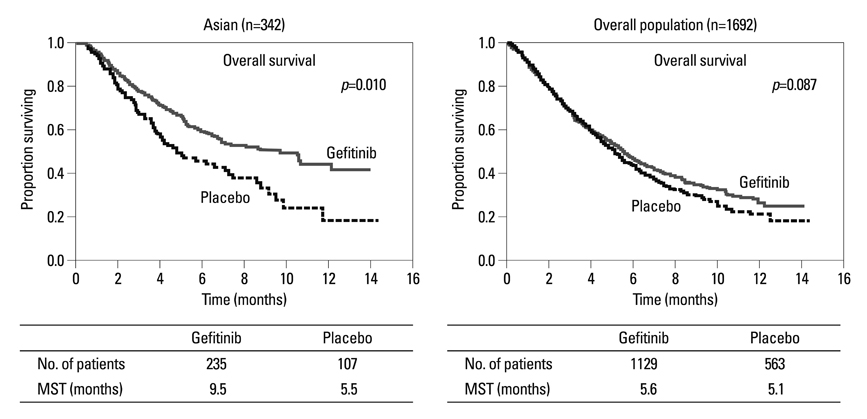
Article34. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005. 366:1527–1537.
Article35. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.
Article36. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.
Article37. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005. 23:2513–2520.
Article38. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005. 23:6829–6837.
Article39. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.
Article40. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010. 362:2380–2388.
Article41. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010. 11:121–128.
Article42. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011. 12:735–742.
Article43. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012. 13:239–246.44. Yang JCH, Schuler MH, Yamamoto N, O'Byrne KJ, Hirsh V, Mok T, et al. LUX-Lung3: a randomized, open-label phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. ASCO 2012. J Clin Oncol. 2012. 30:suppl. Abstract LBA7500.45. Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002. 297:63–64.46. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000. 20:1245–1259.
Article47. Kishi K, Nakata K, Yoshimura K. Efficacy of gefitinib in a patient with lung cancer associated with idiopathic pulmonary fibrosis. J Thorac Oncol. 2006. 1:733–734.
Article48. Takano T, Ohe Y, Kusumoto M, Tateishi U, Yamamoto S, Nokihara H, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004. 45:93–104.
Article49. Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008. 177:1348–1357.
Article50. Jones SJ, Milenkova T. ILD during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2010. 5:1877–1878.
Article51. Lind JS, Smit EF, Grünberg K, Senan S, Lagerwaard FJ. Fatal interstitial lung disease after erlotinib for non-small cell lung cancer. J Thorac Oncol. 2008. 3:1050–1053.
Article52. Liu V, White DA, Zakowski MF, Travis W, Kris MG, Ginsberg MS, et al. Pulmonary toxicity associated with erlotinib. Chest. 2007. 132:1042–1044.
Article53. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009. 373:1525–1531.
Article54. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011. 29:3968–3976.
Article55. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.
Article56. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009. 10:25–34.
Article57. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011. 47:2117–2127.
Article58. Nishina T, Hirashima T, Sugio K, Muro K, Akinaga S, Maeda H, et al. The effect of CYP2C19 polymorphism on the tolerability of ARQ 197: results from phase I trial in Japanese patients with metastatic solid tumors. J Clin Oncol. 2011. 29:2516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Challenges in the Use of Targeted Therapies in Non–Small Cell Lung Cancer
- Beyond angiogenesis blockade: targeted therapy for advanced cervical cancer
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Cytotoxic Chemotherapy for Non-small Cell Lung Cancer
- Present Status and Problems on Molecular Targeted Therapy of Cancer