Korean Circ J.
2008 Sep;38(9):441-445. 10.4070/kcj.2008.38.9.441.
The Clinical Effects of Cilostazol on Atherosclerotic Vascular Disease
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Yeungnam University Hospital, Daegu, Korea. pjs@med.yu.ac.kr
- KMID: 1776343
- DOI: http://doi.org/10.4070/kcj.2008.38.9.441
Abstract
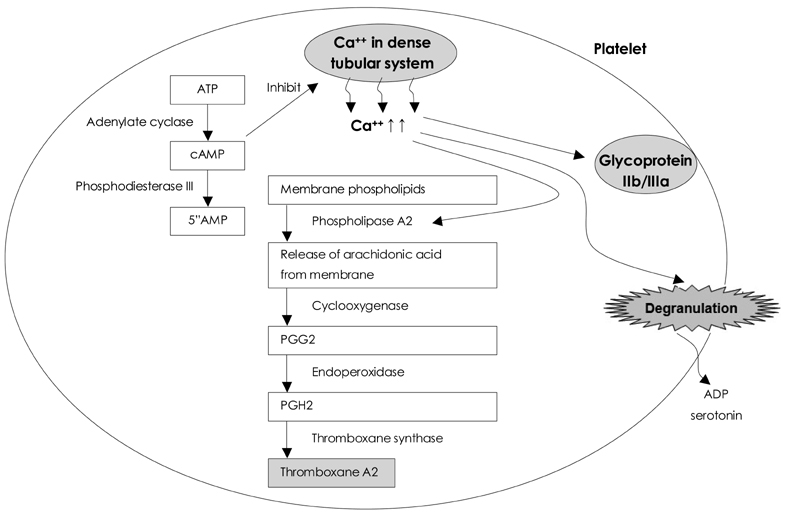
- Cilostazol inhibits phosphodiesterase III (PDE III), which is predominantly distributed to and regulates physiologic responses in platelets, cardiac muscle cells, vascular smooth muscle cells, and adipose cells. Clinically, it is well known as an antiplatelet agent that inhibits the platelet aggregation normally induced by collagen, 5'-adenosine diphosphase (ADP), epinephrine, and arachidonic acid. It also has pleotropic effects, including the prevention of restenosis after angioplasty and the promotion of peripheral vascular flow in patients with peripheral vascular diseases. In the drug-eluting stent era, it has emerged as an effective post-intervention anti-atherothrombotic agent and a useful agent for therapy in diabetic patients. The aim of this study was to review the mechanisms of action and clinical trial results associated with cilostazol in cardiovascular disease patients.
Keyword
MeSH Terms
-
Angioplasty
Arachidonic Acid
Blood Platelets
Cardiovascular Diseases
Collagen
Coronary Restenosis
Cyclic Nucleotide Phosphodiesterases, Type 3
Drug-Eluting Stents
Epinephrine
Humans
Muscle, Smooth, Vascular
Myocytes, Cardiac
Peripheral Vascular Diseases
Platelet Aggregation
Tetrazoles
Thrombosis
Vascular Diseases
Arachidonic Acid
Collagen
Cyclic Nucleotide Phosphodiesterases, Type 3
Epinephrine
Tetrazoles
Figure
Cited by 1 articles
-
Comparative Study of
Ex Vivo Antiplatelet Activity of Aspirin and Cilostazol in Patients with Diabetes and High Risk of Cardiovascular Disease
Sangmo Hong, Woo Je Lee, Cheol-Young Park
Endocrinol Metab. 2022;37(2):233-242. doi: 10.3803/EnM.2021.1353.
Reference
-
1. Schrör K. The pharmacology of cilostazol. Diabetes Obes Metab. 2002. 4:Suppl 2. S14–S19.2. Nakamura N, Osawa H, Yamabe H, Okumura K, Hamazaki T. Effects of cilostazol on lipid and fatty acid metabolism. Clin Exp Med. 2005. 4:170–173.3. Weiss B, Hait WN. Selective cyclic nucleotide phosphodiesterase inhibitors as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 1977. 17:441–477.4. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992. 20:900–906.5. Okuda Y, Kimura Y, Yamashita K. Cilostazol. Cardiovasc Drug Rev. 1993. 11:451–465.6. Lee KJ, Yun SW, Kim SW, Kim TH, Kim CJ, Ryu WS. The effects of cilostazol on proliferation of vascular smooth muscle cells and expression of iNOS and p21. Korean Circ J. 2004. 34:500–506.7. Inoue T, Uchida T, Sakuma M, et al. Cilostazol inhibits leukocyte integrin Mac-1, leading to a potential reduction in restenosis after coronary stent implantation. J Am Coll Cardiol. 2004. 44:1408–1414.8. Kim MJ, Lee JH, Park SY, et al. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006. 54:261–267.9. Nishio Y, Kashiwagi A, Takahara N, Hidaka H, Kikkawa R. Cilostazol, a cAMP phosphodiesterase inhibitor, attenuates the production of monocyte chemoattractant protein-1 in response to tumor necrosis factor-alpha in vascular endothelial cells. Horm Metab Res. 1997. 29:491–495.10. Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis. 2006. 189:350–357.11. Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985. 35:1144–1149.12. Hong KW, Lee JH, Kima KY, Park SY, Lee WS. Cilostazol: therapeutic potential against focal cerebral ischemic damage. Curr Pharm Des. 2006. 12:565–573.13. Tsuchikane E, Takeda Y, Nasu K, Awata N, Kobayashi T. Balloon angioplasty plus cilostazol administration versus primary stenting of small coronary artery disease: final results of COMPASS. Catheter Cardiovasc Interv. 2004. 63:44–51.14. Bae Y, Jeong MH, Kim NH, et al. The effects of anti-platelet agents in preventing coronary stent restenosis. Korean Circ J. 1999. 29:357–365.15. Zhang Z, Foster JK, Kolm P, et al. Reduced 6-month resource use and costs associated with cilostazol in patients after successful coronary stent implantation. Am Heart J. 2006. 152:770–776.16. Tanimoto S, Daemen J, Tsuchida K, et al. Two-year clinical outcome after coronary stenting of small vessels using 2.25-mm sirolimus-and paclitaxel-eluting stents. Catheter Cardiovasc Interv. 2007. 69:94–103.17. Ahn YK, Jeong MH, Jeong JW, et al. Randomized comparison of cilostazol vs clopidogrel after drug-eluting stenting in diabetic patients: clilostazol for diabetic patients in drug-eluting stent (CIDES) trial. Circ J. 2008. 72:35–39.18. Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial. J Am Coll Cardiol. 2008. 51:1181–1187.19. Lee SW, Park SW, Kim YH, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am J Cardiol. 2007. 100:1103–1108.20. Kim SM, Kim DI, Cho HJ, et al. Effects of cilostazol on the drug-eluting stent in native coronary arteries. Korean Circ J. 2007. 37:304–311.21. Mishkel GJ, Aguirre FV, Ligon RW, Rocha-Singh KJ, Lucore CL. Clopidogrel as adjunctive antiplatelet therapy during coronary stenting. J Am Coll Cardiol. 1999. 34:1884–1890.22. Lee SW, Park SW, Hong MK, et al. Comparison of cilostazol and clopidogrel after successful coronary stenting. Am J Cardiol. 2005. 95:859–862.23. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005. 293:2126–2130.24. Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004. 109:3171–3175.25. Shim CY, Yoon SJ, Park S, et al. The clopidogrel resistance can be attenuated with triple antiplatelet therapy in patients undergoing drug-eluting stents implantation. Int J Cardiol. 2008. [Epub ahead of print].26. Kawamura K, Watanabe K, Kimura Y. Effect of cilostazol, a new antithrombotic drug, on cerebral circulation. Arzneimittelforschung. 1985. 35:1149–1154.27. Ohashi S, Iwatani M, Hyakuna Y, Morioka Y. Thermographic evaluation of the hemodynamic effect of the antithrombotic drug cilostazol in peripheral arterial occlusion. Arzneimittelforschung. 1985. 35:1203–1208.28. Beebe HG, Dawson DL, Cutler BS, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999. 159:2041–2050.29. Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines(8th edition). Chest. 2008. 133:6 Suppl. 815S–843S.30. Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the Cilostazol Stroke Prevention Study. Atheroscler Suppl. 2005. 6:33–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Effect of Cilostazol in Diabetic Patients with Peripheral Vascular Disease
- The Effects of Cilostazol on Proliferation of Vascular Smooth Muscle Cells and Expression of iNOS and p21
- Risk Factors of the Atherosclerotic Peripheral Vascular Disease(PVD)
- Ginkgo biloba extract (GbE) enhances the anti-atherogenic effect of cilostazol by inhibiting ROS generation
- Comparative Study of Ex Vivo Antiplatelet Activity of Aspirin and Cilostazol in Patients with Diabetes and High Risk of Cardiovascular Disease