Korean Circ J.
2009 Jan;39(1):16-20. 10.4070/kcj.2009.39.1.16.
Effect of Cardiac Nerve Growth Factor in Acute Myocardial Infarction in Human
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. oys@catholic.ac.kr
- KMID: 1769508
- DOI: http://doi.org/10.4070/kcj.2009.39.1.16
Abstract
-
BACKGROUND AND OBJECTIVES: It has been demonstrated that the concentration of plasma nerve growth factor (NGF) effects nerve sprouting. In addition, the relationship between plasma NGF concentration and the occurrence of ventricular tachyarrhythmia (VT) has been reported in animal models of myocardial infarction (MI). However, the causal relationship between NGF and VT remains unclear in humans. The aim of the current study was to determine whether NGF is increased in patients with MI. In addition, the relationship between the concentration of plasma NGF and the inducibility of VT was evaluated.
SUBJECTS AND METHODS
We studied 15 patients with stable angina pectoris (SA) and 30 patients with an acute MI (AMI). The patients in the AMI group were divided into VT occurrence (n=14) and non-VT occurrence groups (n=16). Thirty-four patients suspected to have VT underwent programmed electrical stimulation (PES) and were divided into an idiopathic VT group (n=24) and an induced VT with PES {healthy control (C) group; n=10}. Plasma NGF concentrations were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS
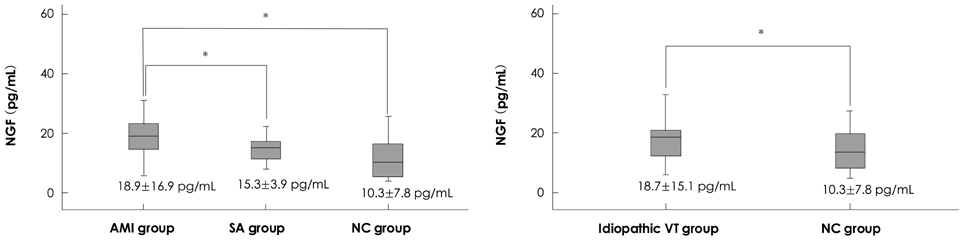
The plasma concentrations of the AMI group were significantly increased compared to the C group {median (interquartile range), 18.9 (8.7) vs. 10.3 (12.5) pg/mL, p<0.05} and the patients with SA {18.9 (8.7) vs. 15.1 (6.7) pg/mL, p<0.05}, but not significantly different from those in the idiopathic VT group {median (interquartile range), 18.9 (8.7) vs. 18.7 (8.5) pg/mL, p=0.89}. There was no significant difference in the plasma NGF concentrations between the C and SA groups {10.3 (12.5) vs. 15.1 (6.7) pg/mL, p=0.18}. In the AMI patients, there was no significant difference in the plasma NGF concentrations between patients with VT and those without VT {18.5 (6.7) vs. 21.2 (10.2) pg/mL, p=0.25}.
CONCLUSION
The plasma NGF concentrations were increased in patients with an AMI compared to patients with SA and Cs.
Keyword
MeSH Terms
Figure
Reference
-
1. Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000. 101:1960–1969.2. Miyauchi Y, Zhou S, Okuyama Y, et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003. 108:360–366.3. Zhou S, Cao JM, Tebb ZD, et al. Modulation of QT interval by cardiac sympathetic nerve sprouting and the mechanisms of ventricular arrhythmia in a canine model of sudden cardiac death. J Cardiovasc Electrophysiol. 2001. 12:1068–1073.4. Chang CM, Wu TJ, Zhou S, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001. 103:22–25.5. Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000. 86:816–821.6. Oh YS, Kim JH, Choi YS, et al. Arrhythmogenic gene change and nerve sprouting after acute myocardial infarction in mice. Korean Circ J. 2007. 37:399–407.7. Kim DH, Shin DG, Jo IH, et al. The significance of cardiac sympathetic nerve imaging with 123I-metaiodobenzylguanidine for predicting the occurrence of ventricular tachycardia in patients with idiopathic dilated cardiomyopathy. Korean Circ J. 2007. 37:510–516.8. Derby A, Engleman VW, Frierdich GE, Neises G, Rapp SR, Roufa DG. Nerve growth factor facilitates regeneration across nerve gaps: morphological and behavioral studies in rat sciatic nerve. Exp Neurol. 1993. 119:176–191.9. Edwards RH, Rutter WJ, Hanahan D. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell. 1989. 58:161–170.10. Levi-Montalcini R. The nerve growth factor: its role in growth, differentiation and function of the sympathetic adrenergic neuron. Prog Brain Res. 1976. 45:235–258.11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003. 26:Suppl 1. S5–S20.12. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003. 42:1206–1252.13. Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.14. Warnick GR, Knopp RH, Fitzppatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem. 1990. 36:15–19.15. Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995. 92:8031–8035.16. Kotlyar AA, Vered Z, Goldberg I, et al. Insulin-like growth factor I and II preserve myocardial structure in postinfarct swine. Heart. 2001. 86:693–700.17. Fuchs M, Hilfiker A, Kaminski K, et al. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J. 2003. 17:2118–2120.18. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004. 94:1543–1553.19. Gwechenberger M, Mendoza LH, Youker KA, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999. 99:546–551.20. Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab. 2000. 71:418–435.21. Oh YS, Jong AY, Kim DT, et al. Spatial distribution of nerve sprouting after myocardial infarction in mice. Heart Rhythm. 2006. 3:728–736.22. Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 2005. 102:169–171.23. Lee MY. Brugada syndrome. Korean Circ J. 2002. 32:461–466.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aconitine Intoxication Misdiagnosed as Acute Myocardial Infarction
- Cardiac arrest due to an unexpected acute myocardial infarction during head and neck surgery: A case report
- Invasive Treatment of Acute Myocardial Infarction: What is the Optimal Therapy for Acute Myocardial Infarction?
- A Case of Pericardial Effusion after Acute Myocardial Infarction
- Left Side Otalgia Caused by Acute Myocardial Infarction