Korean J Physiol Pharmacol.
2012 Jun;16(3):205-209. 10.4196/kjpp.2012.16.3.205.
Development of Estimation Methods of Skin Oxidation and Evaluation of Anti-Oxidative Effects of Genistein in Topical Formulations
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. jaehwi@cau.ac.kr
- 2Department of Physiology and Biophysics, Inha University College of Medicine, Incheon 402-752, Korea.
- 3Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 1768015
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.205
Abstract
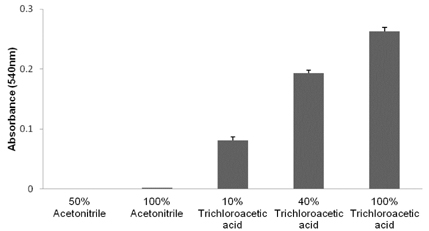
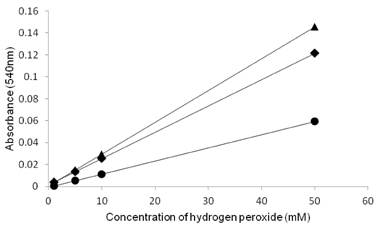
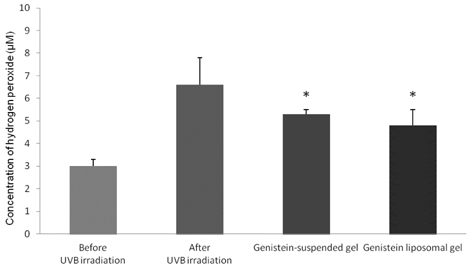
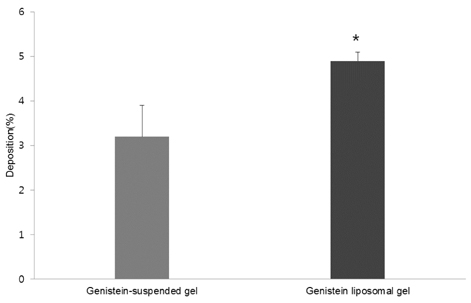
- The objective of the present study was to establish the method of measurement of hydrogen peroxide and to estimate the anti-oxidative effect of genistein in the skin. UVB induced skin oxidation and anti-oxidative effect of genistein formulations were evaluated by determining levels of hydrogen peroxide. The mechanism involved in the determination of hydrogen peroxide is based on a color reaction between ferric ion (Fe3+) and xylenol orange, often called FOX assay and subsequent monitoring of absorbance values of the reactant at 540 nm. The reaction was to some extent pH-dependent and detection sensitivity was greatest at pH 1.75. Genistein liposomal gel demonstrated better anti-oxidative effect with regard to lowering hydrogen peroxide levels elevated by UVB irradiation compared to genistein-suspended gel. A linear relationship has been observed between anti-oxidative effect of genistein and drug deposition in the skin tissue. Genistein liposomal gel resulting in the localization of the drug in the deeper skin led to improved anti-oxidative effect compared to genistein gel. The suggested method for evaluation of oxidation of the skin can be used as a tool to screen effective anti-oxidative agents and their delivery systems acting on the skin.
Keyword
MeSH Terms
Figure
Reference
-
1. Barnes S, Grubbs C, Setchell KD, Carlson J. Soybeans inhibit mammary tumors in models of breast cancer. Prog Clin Biol Res. 1990. 347:239–253.2. Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignani F, Riccardi C, Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res. 1994. 18:431–439.3. Zhou JR, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. Inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998. 58:5231–5238.4. Booth C, Hargreaves DF, Hadfield JA, McGown AT, Potten CS. Isoflavones inhibit intestinal epithelial cell proliferation and induce apoptosis in vitro. Br J Cancer. 1999. 80:1550–1557.5. Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE). Phytother Res. 1999. 13:601–608.6. Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004. 43:326–335.7. Andre P. Hyaluronic acid and its use as a "rejuvenation" agent in cosmetic dermatology. Semin Cutan Med Surg. 2004. 23:218–222.8. Wei H, Zhang X, Wang Y, Lebwohl M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002. 185:21–29.9. Södergren E, Nourooz-Zadeh J, Berglund L, Vessby B. Re-evaluation of the ferrous oxidation in xylenol orange assay for the measurement of plasma lipid hydroperoxides. J Biochem Biophys Methods. 1998. 37:137–146.10. Jiang ZY, Woollard AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990. 268:69–71.11. Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clin Chim Acta. 2003. 337:147–152.12. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005. 38:1103–1111.13. Masaki H, Sakurai H. Increased generation of hydrogen peroxide possibly from mitochondrial respiratory chain after UVB irradiation of murine fibroblasts. J Dermatol Sci. 1997. 14:207–216.14. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003. 91:179–194.15. Podhaisky HP, Riemschneider S, Wohlrab W. UV light and oxidative damage of the skin. Pharmazie. 2002. 57:30–33.16. Kwon SH, Kang MJ, Huh JS, Ha KW, Lee JR, Lee SK, Lee BS, Han IH, Lee MS, Lee MW, Lee J, Choi YW. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm. 2007. 337:148–154.17. Niemiec SM, Ramachandran C, Weiner N. Influence of nonionic liposomal composition on topical delivery of peptide drugs into pilosebaceous units: an in vivo study using the hamster ear model. Pharm Res. 1995. 12:1184–1188.18. Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994. 76:215–224.19. Iwai I, Hatao M, Naganuma M, Kumano Y, Ichihashi M. UVA-induced immune suppression through an oxidative pathway. J Invest Dermatol. 1999. 112:19–24.20. Verma DD, Verma S, Blume G, Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm. 2003. 55:271–277.21. Kirjavainen M, Urtti A, Valjakka-Koskela R, Kiesvaara J, Mönkkönen J. Liposome-skin interactions and their effects on the skin permeation of drugs. Eur J Pharm Sci. 1999. 7:279–286.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidation and anti-inflammatory effects of gamma-irradiated silk sericin and fibroin in H 2O2-induced HaCaT Cell
- Preventive Effects of Multi-Lamellar Emulsion on Low Potency Topical Steroid Induced Local Adverse Effect
- The Effects of Artificial Tear Formulations and Anti-inflammatory Agents on the Cultured Keratocytes of Rabbit
- In Vivo Study for Percutaneous Absorption of Topical Corticosteroids using The Peel-Off Type Pack as a Vehicle
- Proper use of topical corticosteroids