Korean J Physiol Pharmacol.
2023 Jan;27(1):105-112. 10.4196/kjpp.2023.27.1.105.
Antioxidation and anti-inflammatory effects of gamma-irradiated silk sericin and fibroin in H 2O2-induced HaCaT Cell
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Jeonbuk National University, Iksan 54596, Korea
- KMID: 2537508
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.105
Abstract
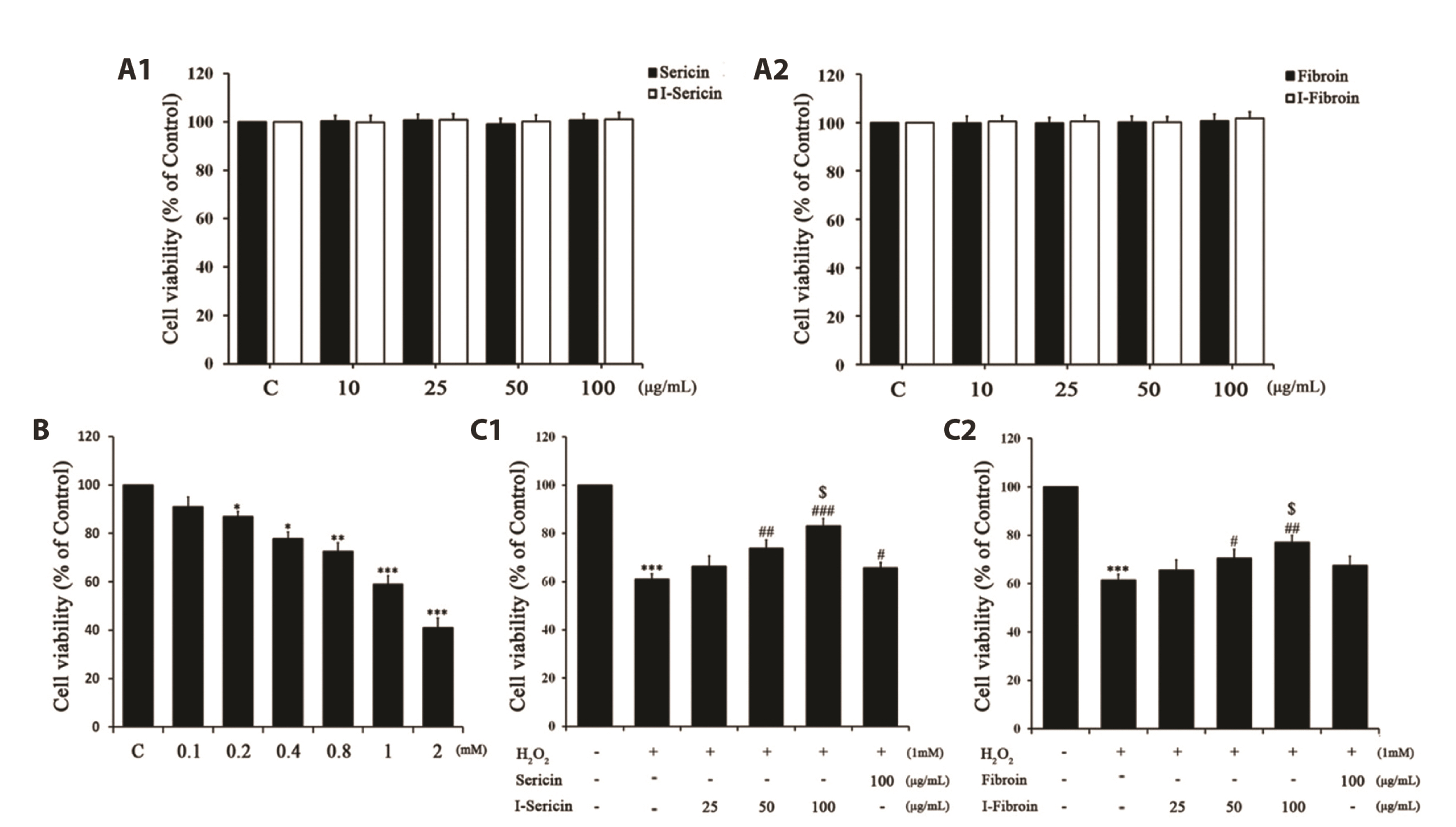
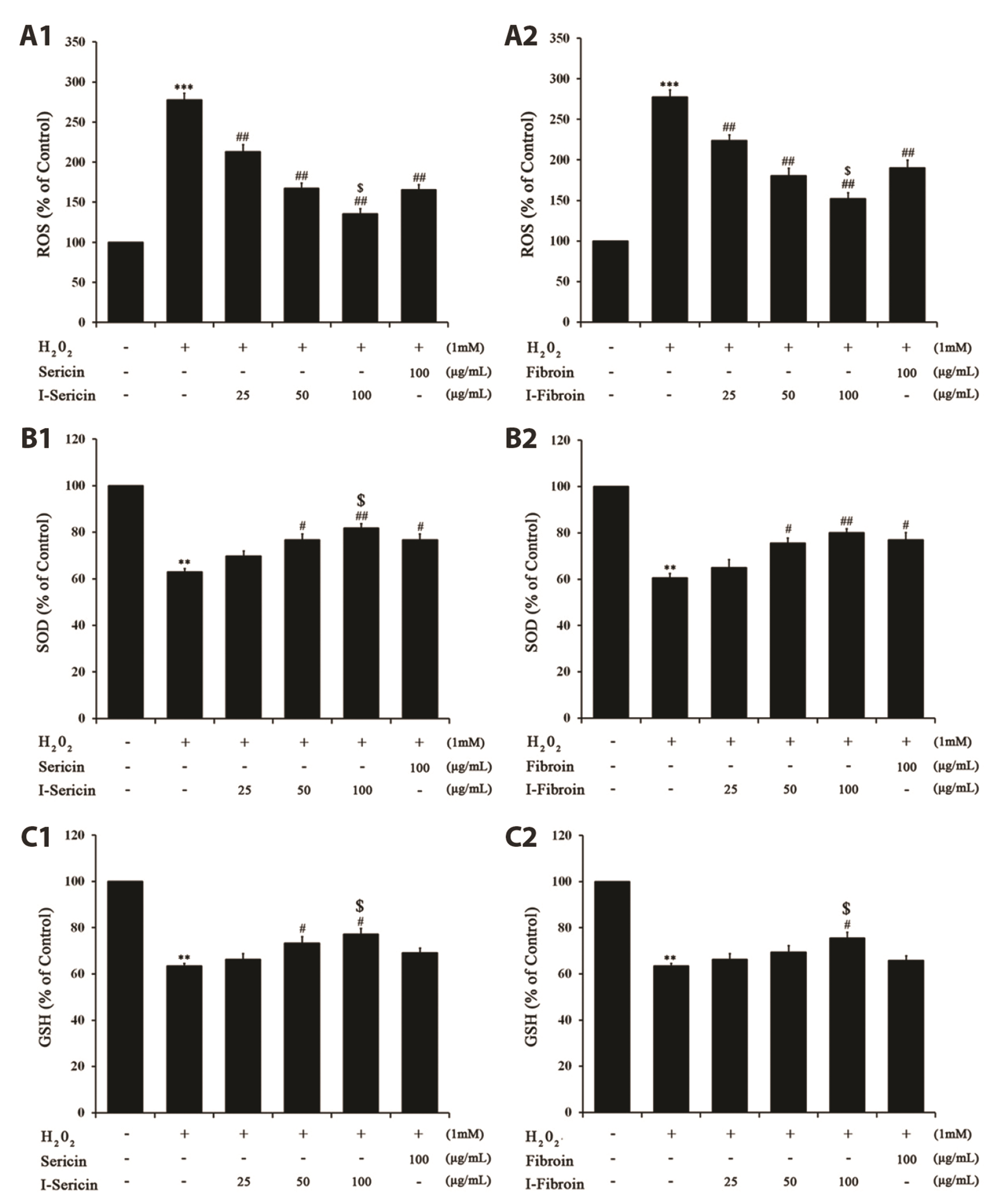
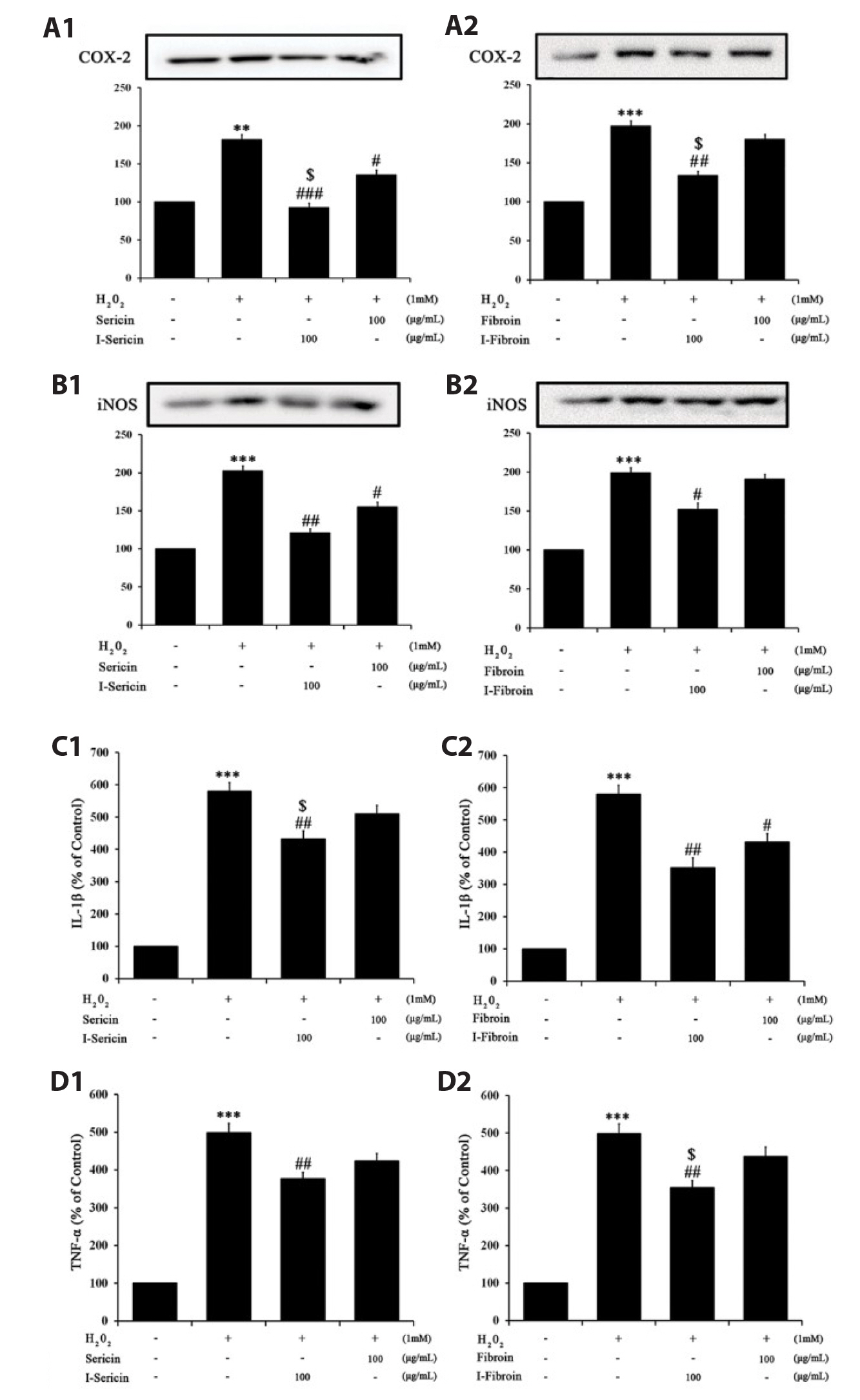
- Oxidative stress in skin cells can induce the formation of reactive oxygen species (ROS), which are critical for pathogenic processes such as immunosuppression, inflammation, and skin aging. In this study, we confirmed improvements from gamma-irradiated silk sericin (I-sericin) and gamma-irradiated silk fibroin (I-fibroin) to skin cells damaged by oxidative stress. We found that I-sericin and I-fibroin effectively attenuated oxidative stress-induced ROS generation and decreased oxidative stress-induced inflammatory factors COX-2, iNOS, tumor necrosis factor-α, and interleukin-1β compared to the use of non-irradiated sericin or fibroin. I-sericin and Ifibroin effects were balanced by competition with skin regenerative protein factors reacting to oxidative stress. Taken together, our results indicated that, compared to non-irradiated sericin or fibroin, I-sericin, and I-fibroin had anti-oxidation and antiinflammation activity and protective effects against skin cell damage from oxidative stress. Therefore, gamma-irradiation may be useful in the development of cosmetics to maintain skin health.
Figure
Reference
-
1. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. 2002; Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 138:1462–1470. DOI: 10.1001/archderm.138.11.1462. PMID: 12437452.2. Sies H. 1997; Oxidative stress: oxidants and antioxidants. Exp Physiol. 82:291–295. DOI: 10.1113/expphysiol.1997.sp004024. PMID: 9129943. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030894162&origin=inward.3. Briganti S, Picardo M. 2003; Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 17:663–669. DOI: 10.1046/j.1468-3083.2003.00751.x. PMID: 14761133. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0242624562&origin=inward.4. Svobodová A, Walterová D, Psotová J. 2006; Influence of silymarin and its flavonolignans on H2O2-induced oxidative stress in human keratinocytes and mouse fibroblasts. Burns. 32:973–979. DOI: 10.1016/j.burns.2006.04.004. PMID: 17011711. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33750480203&origin=inward.5. Li BC, Zhang SQ, Dan WB, Chen YQ, Cao P. 2007; Expression in Escherichia coli and purification of bioactive antibacterial peptide ABP-CM4 from the Chinese silk worm, Bombyx mori. Biotechnol Lett. 29:1031–1036. DOI: 10.1007/s10529-007-9351-4. PMID: 17375264. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34249002818&origin=inward.6. Manosroi A, Boonpisuttinant K, Winitchai S, Manosroi W, Manosroi J. 2010; Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori). Pharm Biol. 48:855–860. DOI: 10.3109/13880200903300212. PMID: 20673171. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77955215561&origin=inward.7. Martínez-Mora C, Mrowiec A, García-Vizcaíno EM, Alcaraz A, Cenis JL, Nicolás FJ. 2012; Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS One. 7:e42271. DOI: 10.1371/journal.pone.0042271. PMID: 22860103. PMCID: PMC3409175. PMID: b530bd87636d4b2eb98669ebc01af49d. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864451851&origin=inward.8. Vepari C, Kaplan DL. 2007; Silk as a biomaterial. Prog Polym Sci. 32:991–1007. DOI: 10.1016/j.progpolymsci.2007.05.013. PMID: 19543442. PMCID: PMC2699289. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34547602612&origin=inward.9. Meinel L, Kaplan DL. 2012; Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 64:1111–1122. DOI: 10.1016/j.addr.2012.03.016. PMID: 22522139. PMCID: PMC3719414. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84865304712&origin=inward.10. Zhang YQ. 2002; Applications of natural silk protein sericin in biomaterials. Biotechnol Adv. 20:91–100. DOI: 10.1016/S0734-9750(02)00003-4. PMID: 14538058. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036268771&origin=inward.11. Aramwit P, Towiwat P, ichana T Sr. 2013; Anti-inflammatory potential of silk sericin. Nat Prod Commun. 8:501–504. DOI: 10.1177/1934578X1300800424. PMID: 23738464. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876277755&origin=inward.12. Dash R, Acharya C, Bindu PC, Kundu SC. 2008; Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 41:236–241. DOI: 10.5483/BMBRep.2008.41.3.236. PMID: 18377728. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=42049106232&origin=inward.13. Dash R, Mandal M, Ghosh SK, Kundu SC. 2008; Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol Cell Biochem. 311:111–119. DOI: 10.1007/s11010-008-9702-z. PMID: 18214642. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=41349084565&origin=inward.14. Leskovac A, Joksic G, Jankovic T, Savikin K, Menkovic N. 2007; Radioprotective properties of the phytochemically characterized extracts of Crataegus monogyna, Cornus mas and Gentianella austriaca on human lymphocytes in vitro. Planta Med. 73:1169–1175. DOI: 10.1055/s-2007-981586. PMID: 17764067. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35348903876&origin=inward.15. Harrison K, Were LM. 2007; Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 102:932–937. https://doi.org/10.1016/j.foodchem.2006.06.034. DOI: 10.1016/j.foodchem.2006.06.034. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846205993&origin=inward.16. Mali AB, Khedkar K, Lele SS. 2011; Effect of gamma irradiation on total phenolic content and in vitro antioxidant activity of pomegranate (Punica granatum L.) peels. Food Nutr Sci. 2:428–433. https://doi.org/10.4236/fns.2011.25060. DOI: 10.4236/fns.2011.25060.17. Pricaz M, Uţă AC. 2015; Gamma radiation for improvements in food industry, environmental quality and healthcare. Rom J Biophys. 2:143–162. https://www.rjb.ro/articles/423/2015-2_Pricaz.pdf. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876277755&origin=inward.18. Song IB, Han HJ, Kwon J. 2020; Immune-enhancing effects of gamma-irradiated sericin. Food Sci Biotechnol. 29:969–976. DOI: 10.1007/s10068-020-00734-6. PMID: 32582459. PMCID: PMC7297890. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079441888&origin=inward.19. Furuno K, Akasako T, Sugihara N. 2002; The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull. 25:19–23. DOI: 10.1248/bpb.25.19. PMID: 11824550. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036365330&origin=inward.20. Bae JY, Choi JS, Choi YJ, Shin SY, Kang SW, Han SJ, Kang YH. 2008; (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem Toxicol. 46:1298–1307. DOI: 10.1016/j.fct.2007.09.112. PMID: 18226437. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39249085230&origin=inward.21. Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. 1995; Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 105:285–290. DOI: 10.1111/1523-1747.ep12318471. PMID: 7543550. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029154783&origin=inward.22. Lu CY, Lee HC, Fahn HJ, Wei YH. 1999; Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 423:11–21. DOI: 10.1016/S0027-5107(98)00220-6. PMID: 10029667. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0002026429&origin=inward.23. Ozben T. 2007; Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 96:2181–2196. DOI: 10.1002/jps.20874. PMID: 17593552. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34748858784&origin=inward.24. Ahn HJ, Kim JH, Kim JK, Kim DH, Yook HS, Byun MW. 2005; Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.). Food Chem. 89:589–597. https://doi.org/10.1016/j.foodchem.2004.03.029. DOI: 10.1016/j.foodchem.2004.03.029. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=4544307471&origin=inward.25. Lee SL, Lee MS, Song KB. 2005; Effect of gamma-irradiation on the physicochemical properties of gluten films. Food Chem. 92:621–625. https://doi.org/10.1016/j.foodchem.2004.08.023. DOI: 10.1016/j.foodchem.2004.08.023. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17444403227&origin=inward.26. el-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. 2002; Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 147:230–243. DOI: 10.1046/j.1365-2133.2002.04871.x. PMID: 12174092. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036380879&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The optimal scaffold for silk sericin‑based bone graft: collagen versus gelatin
- Dietary effect of silk protein on epidermal levels of free sphingoid bases and phosphate metabolites in NC/Nga mice
- Anti-inflammatory Effect of Curcumin on UVB-induced Inflammatory Cytokines in HaCaT Cells
- Dietary Effect of Silk Protein Sericin or Fibroin on Plasma and Epidermal Amino Acid Concentration of NC/Nga Mice
- Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice