Clin Orthop Surg.
2011 Sep;3(3):191-201. 10.4055/cios.2011.3.3.191.
Grafting Using Injectable Calcium Sulfate in Bone Tumor Surgery: Comparison with Demineralized Bone Matrix-based Grafting
- Affiliations
-
- 1Orthopedic Oncology Clinic, National Cancer Center, Goyang, Korea.
- 2Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, Korea. smilecsw@gmail.com
- KMID: 1743909
- DOI: http://doi.org/10.4055/cios.2011.3.3.191
Abstract
- BACKGROUND
Injectable calcium sulfate is a clinically proven osteoconductive biomaterial, and it is an injectable, resorbable and semi-structural bone graft material. The purpose of this study was to validate the clinical outcomes of injectable calcium sulfate (ICS) grafts as compared with those of a demineralized bone matrix (DBM)-based graft for filling in contained bony defects created by tumor surgery.
METHODS
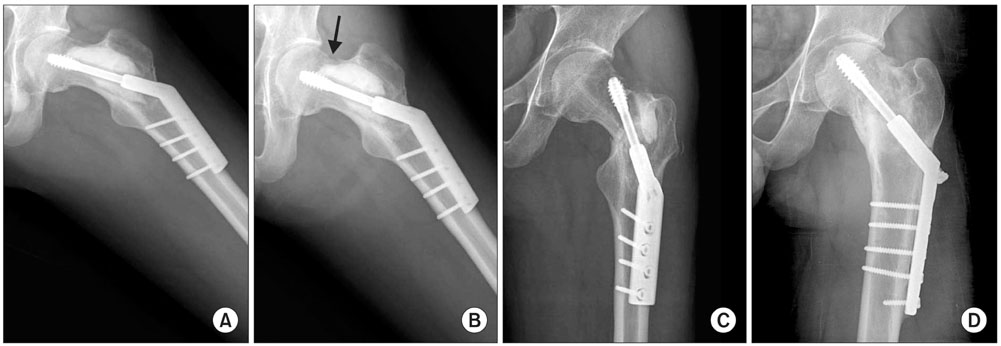
Fifty-six patients (41 males and 15 females) with various bone tumors and who were surgically treated between September 2003 and October 2007 were included for this study. The patients were randomly allocated into two groups, and either an ICS graft (28 patients) or a DBM-based graft (28 patients) was implanted into each contained defect that was developed by the surgery. The radiographic outcomes were compared between the two groups and various clinical factors were included for the statistical analysis.
RESULTS
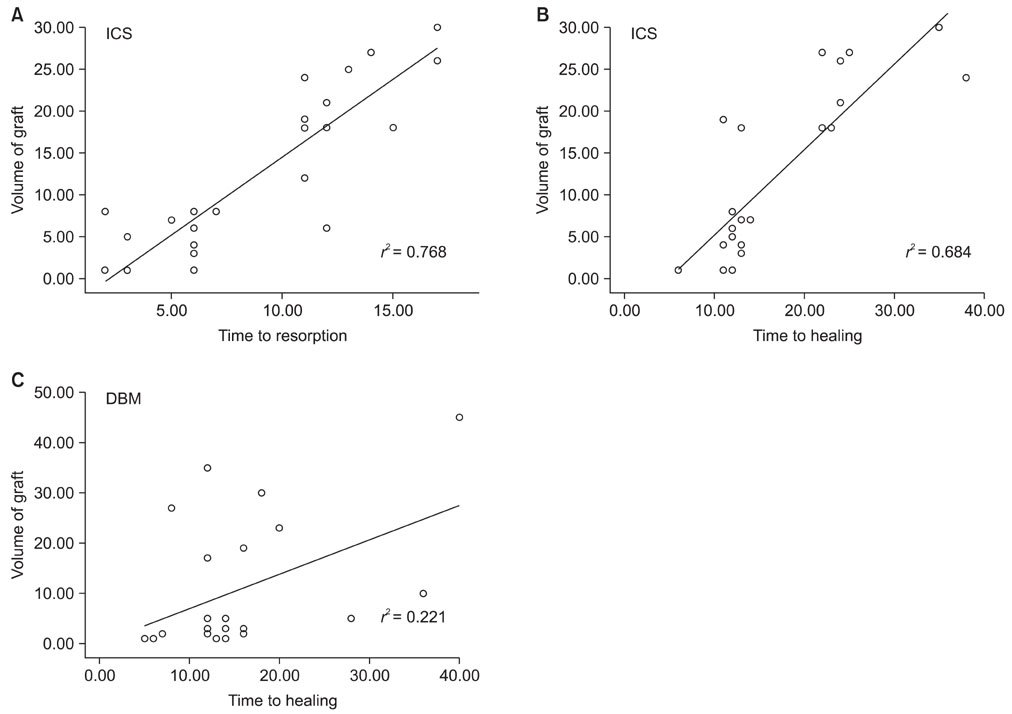
When one case with early postoperative pathologic fracture in the DBM group was excluded, the overall success rates of the ICS and DBM grafting were 85.7% (24/28) and 88.9% (24/27) (p > 0.05), respectively. The average time to complete healing was 17.3 weeks in the ICS group and 14.9 weeks in the DBM group (p > 0.05). Additionally, the ICS was completely resorbed within 3 months, except for one case.
CONCLUSIONS
Although the rate of resorption of ICS is a concern, the injectable calcium sulfate appears to be a comparable bone graft substitute for a DBM-based graft, with a lower cost, for the treatment of the bone defects created during surgery for various bone tumors.
MeSH Terms
-
Absorbable Implants
Adolescent
Adult
Biocompatible Materials/*administration & dosage
Bone Demineralization Technique
Bone Matrix/*transplantation
Bone Neoplasms/radiography/surgery/*therapy
Bone Substitutes/*administration & dosage
Calcium Sulfate/*administration & dosage
Child
Child, Preschool
Curettage
Female
Humans
Infant
Injections
Male
Middle Aged
Wound Healing
Young Adult
Figure
Reference
-
1. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002. (395):44–52.
Article2. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989. 3(3):192–195.
Article3. Dreesmann H. Ueber knochenplombierung. Beitr Klin Chir. 1892. 9:804–810.4. Blaha JD. Calcium sulfate bone-void filler. Orthopedics. 1998. 21(9):1017–1019.
Article5. Walsh WR, Morberg P, Yu Y, et al. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res. 2003. (406):228–236.
Article6. Yu B, Han K, Ma H, et al. Treatment of tibial plateau fractures with high strength injectable calcium sulphate. Int Orthop. 2009. 33(4):1127–1133.
Article7. Yi X, Wang Y, Lu H, Li C, Zhu T. Augmentation of pedicle screw fixation strength using an injectable calcium sulfate cement: an in vivo study. Spine (Phila Pa 1976). 2008. 33(23):2503–2509.
Article8. Oh JH, Yoon PW, Lee SH, Cho HS, Kim WS, Kim HS. Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant. Int Orthop. 2006. 30(6):490–494.
Article9. Belkoff SM, Sanders JC, Jasper LE. The effect of the monomer-to-powder ratio on the material properties of acrylic bone cement. J Biomed Mater Res. 2002. 63(4):396–399.
Article10. Wingerter S, Tucci M, Bumgardner J, Benghuzzi H. Evaluation of short-term healing following sustained delivery of osteoinductive agents in a rat femur drill defect model. Biomed Sci Instrum. 2007. 43:188–193.11. Batinic D, Marusic M, Pavletic Z, et al. Relationship between differing volumes of bone marrow aspirates and their cellular composition. Bone Marrow Transplant. 1990. 6(2):103–107.12. Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000. (376):200–208.
Article13. Kwon JW, Choi JA, Kwack KS, Oh JH, Chung JH, Kang HS. Myxoid chondrosarcoma in the calcaneus: a case report with MR imaging findings. Skeletal Radiol. 2007. 36:Suppl 1. S82–S85.
Article14. Bloemers FW, Blokhuis TJ, Patka P, Bakker FC, Wippermann BW, Haarman HJ. Autologous bone versus calciumphosphate ceramics in treatment of experimental bone defects. J Biomed Mater Res B Appl Biomater. 2003. 66(2):526–531.
Article15. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graftharvest donor site morbidity: a statistical evaluation. Spine (Phila Pa 1976). 1995. 20(9):1055–1060.16. Rohmiller MT, Schwalm D, Glattes RC, Elalayli TG, Spengler DM. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J. 2002. 2(4):255–260.
Article17. Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001. (382):42–50.18. Clayer M. Injectable form of calcium sulphate as treatment of aneurysmal bone cysts. ANZ J Surg. 2008. 78(5):366–370.
Article19. Gitelis S, Piasecki P, Turner T, Haggard W, Charters J, Urban R. Use of a calcium sulfate-based bone graft substitute for benign bone lesions. Orthopedics. 2001. 24(2):162–166.
Article20. Peltier LF. The use of plaster of Paris to fill defects in bone. Clin Orthop. 1961. 21:1–31.21. Peltier LF, Jones RH. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J Bone Joint Surg Am. 1978. 60(6):820–822.
Article22. Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002. 84(3):454–464.
Article23. Hu G, Xiao L, Fu H, Bi D, Ma H, Tong P. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. J Mater Sci Mater Med. 2010. 21(2):627–634.
Article24. Sampath TK, Muthukumaran N, Reddi AH. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987. 84(20):7109–7113.
Article25. Tiedeman JJ, Garvin KL, Kile TA, Connolly JF. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995. 18(12):1153–1158.
Article26. Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder: a radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987. 69(7):984–992.
Article27. Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Joint Surg Am. 2002. 84(6):921–929.
Article28. Iwata H, Sakano S, Itoh T, Bauer TW. Demineralized bone matrix and native bone morphogenetic protein in orthopaedic surgery. Clin Orthop Relat Res. 2002. (395):99–109.
Article29. Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004. 70(1):21–29.
Article30. Takikawa S, Bauer TW, Kambic H, Togawa D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J Biomed Mater Res A. 2003. 65(1):37–42.
Article