Enhanced compatibility and initial stability of Ti6Al4V alloy orthodontic miniscrews subjected to anodization, cyclic precalcification, and heat treatment
- Affiliations
-
- 1Sun Dental Hospital, Daejeon, Korea.
- 2Department of Dental Biomaterials and Institute of Oral Bioscience, Brain Korea 21 Project, School of Dentistry, Chonbuk National University, Jeonju, Korea.
- 3Department of Orthodontics, School of Dentistry, Chonbuk National University, Jeonju, Korea. kjgortho@jbnu.ac.kr
- KMID: 1726418
- DOI: http://doi.org/10.4041/kjod.2014.44.5.246
Abstract
OBJECTIVE
To evaluate the bioactivity, and the biomechanical and bone-regenerative properties of Ti6Al4V miniscrews subjected to anodization, cyclic precalcification, and heat treatment (APH treatment) and their potential clinical use.
METHODS
The surfaces of Ti6Al4V alloys were modified by APH treatment. Bioactivity was assessed after immersion in simulated body fluid for 3 days. The hydrophilicity and the roughness of APH-treated surfaces were compared with those of untreated (UT) and anodized and heat-treated (AH) samples. For in vivo tests, 32 miniscrews (16 UT and 16 APH) were inserted into 16 Wistar rats, one UT and one APH-treated miniscrew in either tibia. The miniscrews were extracted after 3 and 6 weeks and their osseointegration (n = 8 for each time point and group) was investigated by surface and histological analyses and removal torque measurements.
RESULTS
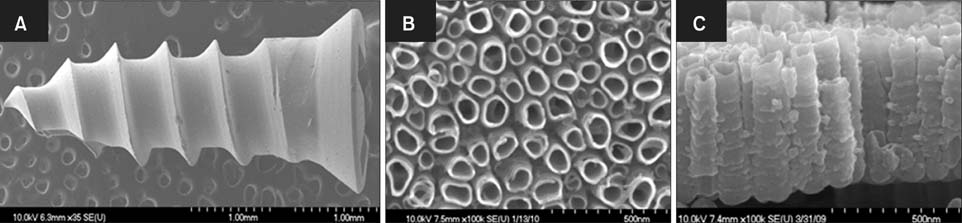
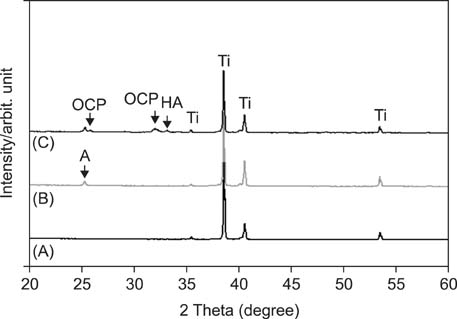
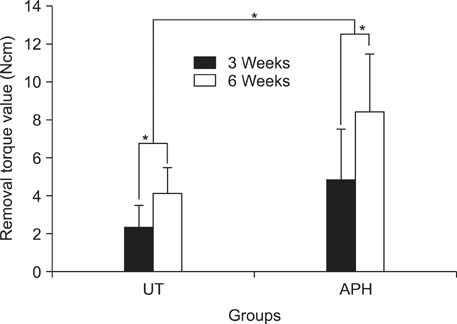
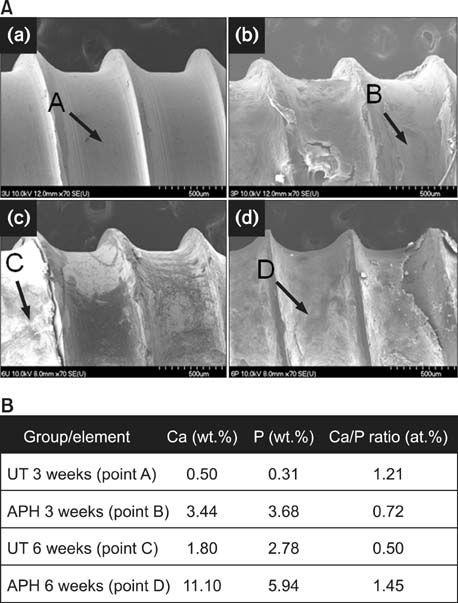
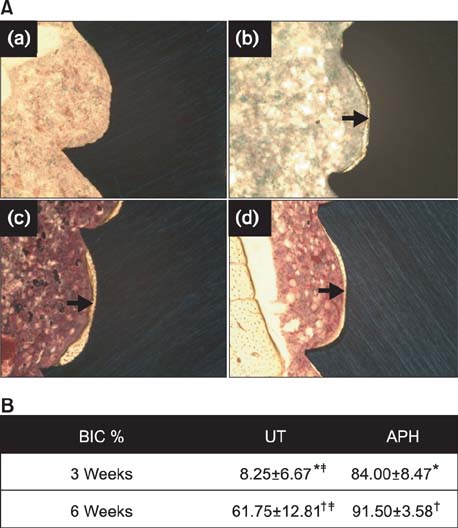
APH treatment formed a dense surface array of nanotubular TiO2 layer covered with a compact apatite-like film. APH-treated samples showed better bioactivity and biocompatibility compared with UT and AH samples. In vivo, APH-treated miniscrews showed higher removal torque and bone-to-implant contact than did UT miniscrews, after both 3 and 6 weeks (p < 0.05). Also, early deposition of densely mineralized bone around APH-treated miniscrews was observed, implying good bonding to the treated surface.
CONCLUSIONS
APH treatment enhanced the bioactivity, and the biomechanical and bone regenerative properties of the Ti6Al4V alloy miniscrews. The enhanced initial stability afforded should be valuable in orthodontic applications.
MeSH Terms
Figure
Cited by 4 articles
-
Bone cutting capacity and osseointegration of surface-treated orthodontic mini-implants
Ho-Young Kim, Sang-Cheol Kim
Korean J Orthod. 2016;46(6):386-394. doi: 10.4041/kjod.2016.46.6.386.Effects of recycling on the biomechanical characteristics of retrieved orthodontic miniscrews
Soon-Dong Yun, Sung-Hwan Choi, Jung-Yul Cha, Hyung-Seog Yu, Kwang-Mahn Kim, Jin Kim, Chung-Ju Hwang
Korean J Orthod. 2017;47(4):238-247. doi: 10.4041/kjod.2017.47.4.238.Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties
Adith Venugopal, Nallal Muthuchamy, Harsh Tejani, Anantha-Iyengar Gopalan, Kwang-Pill Lee, Heon-Jin Lee, Hee Moon Kyung
Korean J Orthod. 2017;47(1):3-10. doi: 10.4041/kjod.2017.47.1.3.The effect of fluoride-containing oral rinses on the corrosion resistance of titanium alloy (Ti-6Al-4V)
Gui-Yue Huang, Heng Bo Jiang, Jung-Yul Cha, Kwang-Mahn Kim, Chung-Ju Hwang
Korean J Orthod. 2017;47(5):306-312. doi: 10.4041/kjod.2017.47.5.306.
Reference
-
1. Ikeda H, Rossouw PE, Campbell PM, Kontogiorgos E, Buschang PH. Three-dimensional analysis of peri-bone-implant contact of rough-surface miniscrew implants. Am J Orthod Dentofacial Orthop. 2011; 139:e153–e163.
Article2. Kim SH, Lee SJ, Cho IS, Kim SK, Kim TW. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009; 79:899–907.
Article3. Chang CS, Lee TM, Chang CH, Liu JK. The effect of microrough surface treatment on miniscrews used as orthodontic anchors. Clin Oral Implants Res. 2009; 20:1178–1184.
Article4. Chaddad K, Ferreira AF, Geurs N, Reddy MS. Influence of surface characteristics on survival rates of mini-implants. Angle Orthod. 2008; 78:107–113.
Article5. Wu X, Deng F, Wang Z, Zhao Z, Wang J. Biomechanical and histomorphometric analyses of the osseointegration of microscrews with different surgical techniques in beagle dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:644–650.
Article6. Suzuki EY, Suzuki B. Placement and removal torque values of orthodontic miniscrew implants. Am J Orthod Dentofacial Orthop. 2011; 139:669–678.
Article7. Wu TY, Kuang SH, Wu CH. Factors associated with the stability of mini-implants for orthodontic anchorage: a study of 414 samples in Taiwan. J Oral Maxillofac Surg. 2009; 67:1595–1599.
Article8. Chen Y, Kang ST, Bae SM, Kyung HM. Clinical and histologic analysis of the stability of microimplants with immediate orthodontic loading in dogs. Am J Orthod Dentofacial Orthop. 2009; 136:260–267.
Article9. Lee NK, Baek SH. Effects of the diameter and shape of orthodontic mini-implants on microdamage to the cortical bone. Am J Orthod Dentofacial Orthop. 2010; 138:8.e1–8.e8.
Article10. Morais LS, Serra GG, Muller CA, Andrade LR, Palermo EF, Elias CN, et al. Titanium alloy mini-implants for orthodontic anchorage: immediate loading and metal ion release. Acta Biomater. 2007; 3:331–339.
Article11. Chen F, Terada K, Hanada K, Saito I. Anchorage effect of osseointegrated vs nonosseointegrated palatal implants. Angle Orthod. 2006; 76:660–665.12. Park IS, Yang EJ, Bae TS. Effect of cyclic precalcification of nanotubular TiO2 layer on the bioactivity of titanium implant. Biomed Res Int. 2013; 2013:293627.13. Nguyen TDT, Park IS, Lee MH, Bae TS. Enhanced biocompatibility of a pre-calcified nanotubular TiO2 layer on Ti-6Al-7Nb alloy. Surf Coatings Technol. 2013; 236:127–134.
Article14. Eaninwene G 2nd, Yao C, Webster TJ. Enhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfaces. Int J Nanomedicine. 2008; 3:257–264.15. Peng L, Mendelsohn AD, LaTempa TJ, Yoriya S, Grimes CA, Desai TA. Long-term small molecule and protein elution from TiO2 nanotubes. Nano Lett. 2009; 9:1932–1936.16. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004; 25:4731–4739.
Article17. Yadav S, Upadhyay M, Liu S, Roberts E, Neace WP, Nanda R. Microdamage of the cortical bone during mini-implant insertion with self-drilling and self-tapping techniques: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2012; 141:538–546.
Article18. Chen Y, Shin HI, Kyung HM. Biomechanical and histological comparison of self-drilling and self-tapping orthodontic microimplants in dogs. Am J Orthod Dentofacial Orthop. 2008; 133:44–50.
Article19. Oh SH, Finõnes RR, Daraio C, Chen LH, Jin S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005; 26:4938–4943.
Article20. Yao C, Perla V, McKenzie JL, Slamovich EB, Webster TJ. Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesion. J Biomed Nanotechnol. 2005; 1:68–73.
Article21. Salata LA, Burgos PM, Rasmusson L, Novaes AB, Papalexiou V, Dahlin C, et al. Osseointegration of oxidized and turned implants in circumferential bone defects with and without adjunctive therapies: an experimental study on BMP-2 and autogenous bone graft in the dog mandible. Int J Oral Maxillofac Surg. 2007; 36:62–71.
Article22. Liou EJ, Pai BC, Lin JC. Do miniscrews remain stationary under orthodontic forces? Am J Orthod Dentofacial Orthop. 2004; 126:42–47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precalcification Treatment of TiO2 Nanotube on Ti-6Al-4V Alloy
- Influence of immediate loading on the removal torque value of mini-screws
- The study of tension characteristics in orthodontic wires
- Bioactivity of precalcified nanotubular TiO2 layer on Ti-6Al-7Nb alloy
- Enhancement of bioactivity and osseointegration in Ti-6Al-4V orthodontic mini-screws coated with calcium phosphate on the TiO 2 nanotube layer