J Korean Acad Nurs.
2009 Dec;39(6):781-787. 10.4040/jkan.2009.39.6.781.
Computerized Measurement for Asthma-Specific Quality of Life: Comparison with a Conventional Paper-and-Pencil Questionnaire
- Affiliations
-
- 1Graduate School of Public Health, Ajou University, Suwon, Korea. ehlee@ajou.ac.kr
- KMID: 1718152
- DOI: http://doi.org/10.4040/jkan.2009.39.6.781
Abstract
- PURPOSE
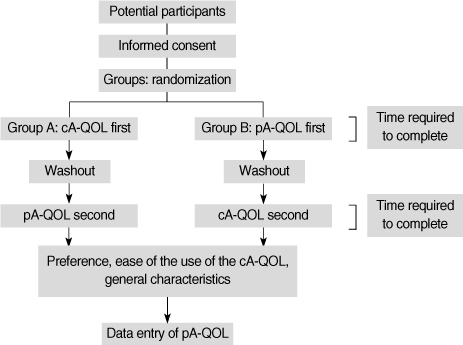
The purpose of this study was to evaluate a computerized touch-screen version of the asthma-specific quality-of-life (cA-QOL) questionnaire against the conventional paper-and-pencil version (pA-QOL) for equivalence, time for completion, user preference, and ease of use. METHODS: A total of 261 patients were recruited. A randomized cross-over design was used. Patients in group A completed the cA-QOL first while waiting to see a physician, and completed the pA-QOL version after seeing the physician. Patients allocated in group B completed these questionnaires in the reverse order. The patients were asked questions about user preference and ease of use of the cA-QOL. The time taken to complete both versions of the questionnaire was measured. RESULTS: Weighted kappa coefficients of all items showed almost perfect agreement. The time required to complete the pA-QOL is faster than the time for cA-QOL. The patients who preferred the cA-QOL were 37.5%, while those who preferred the pA-QOL were 29.9%. Most patients reported that the cA-QOL was "easy" or "very easy" to complete. CONCLUSION: The cA-QOL is the computerized equivalent of the pA-QOL. The findings herein demonstrate that the cA-QOL can be helpful to nurses in busy practices for assessing, collecting, and evaluating their patients' health related quality of life.
Keyword
MeSH Terms
Figure
Reference
-
1. Allenby A, Matthews J, Beresford J, McLachlan SA. The application of computer touch-screen technology in screening for psychosocial distress in an ambulatory oncology setting. European Journal of Cancer Care. 2002. 11:245–253.2. American Psychology Association. Guidelines for computer based tests and interpretation. 1986. Washington DC: Author.3. Armitage P, Berry G. Armitage P, Berry G, editors. Further analysis of categorical data: Kappa measure of agreement. Statistical Methods in Medical Research. 1995. 3rd ed.Malden, MA: Blackwell Science;443–447.4. Bliven BD, Kaufman SE, Spertus JA. Electronic collection of health-related quality of life data: Validity, time benefit, and patient preference. Quality of Life Research. 2001. 10:15–22.5. Cohen J. Statistical Analysis for the Behavioral Sciences. 1987. Rev. ed.Hillsdale, NJ: Lawrence Erlbaum Association.6. Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax. 1992. 47:76–83.7. Kim CW, Chung HW, Shin JI, Bae SW, Park JW, Cho SY, et al. Generic health-related quality of life in patients with bronchial asthma. Korean Journal of Asthma, Allergy and Clinical Immunology. 2002. 22:558–566.8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.9. Lee EH. Touch-screen computerized quality-of-life assessment for patients with cancer. Asian Nursing Research. 2009. 3:41–48.10. Lee EH, Kim SH, Choi JH, Jee YG, Nahm DH, Park HS. Development and evaluation of an asthma-specific quality of life (A-QOL) questionnaire. Journal of Asthma. 2009. 46:716–721.11. Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. Journal of Clinical Epidemiology. 1992. 45:461–472.12. Pocock SJ. Clinical Trials. 1983. Chichester: John Wiley & Sons.13. Pouwer F, Snoek FJ, van der Ploeg HM, Heine RJ, Brand AN. A comparison of the standard and the computerized versions of the Well-Being Questionnaire (WBQ) and the Diabetes Treatment Satisfaction Questionnaire (DTSQ). Quality of Life Research. 1998. 7:33–38.14. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 general health questionnaire to standard paper version. Quality of Life Research. 2002. 11:19–26.15. Schmier JK, Chan KS, Leidy NK. The impact of asthma on health-related quality of life. Journal of Asthma. 1998. 35:585–597.16. Tooth LR, Ottenbacher KJ. The κ statistics in rehabilitation research: An examination. Archives of Physical Medicine and Rehabilitation. 2004. 85:1371–1376.17. Velikova G, Wright EP, Smith AB, Cull A, Gould A, Forman D, et al. Automated collection of quality of life data: A comparison of paper and computer touch-screen questionnaire. Journal of Clinical Oncology. 1999. 17:998–1007.18. Weinberger M, Oddone EZ, Samsa GP, Pamela BL. Are health-related quality of life measures affected by the mode of administration? Journal of Clinical Epidemiology. 1996. 49:135–140.19. Wilson AS, Kitas GD, Karruthers DM, Reay C, Skan J, Harris S, et al. Computerized information-gathering in specialist rheumatology clinics: An initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002. 41:268–273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and multicenter study on Korean pediatric asthma caregiver's quality of life questionnaire (KPACQLQ)
- Development and multicenter study on Korean pediatric asthma quality of life questionnaire (KPAQLQ)
- Utilization of quality of life questionnaire for asthmatics
- The Methods for Foot Function Index and Foot and Ankle Outcome Score Measurement: A Comparison between Paper-and-Pencil Method and Electronic Method
- Symptom Experiences and Quality of Life in People with Asthma