Korean J Radiol.
2013 Aug;14(4):581-588. 10.3348/kjr.2013.14.4.581.
Optimizing the Imaging Protocol for Ex Vivo Coronary Artery Wall Using High-Resolution MRI: An Experimental Study on Porcine and Human
- Affiliations
-
- 1Department of Medical, The General Hospital of Chinese People's Armed Police Forces, Beijing 100039, China.
- 2Department of Radiology, The General Hospital of Chinese People's Armed Police Forces, Beijing 100039, China. litaofeivip@163.com
- 3Department of Pathology, The General Hospital of Chinese People's Armed Police Forces, Beijing 100039, China.
- KMID: 1715762
- DOI: http://doi.org/10.3348/kjr.2013.14.4.581
Abstract
OBJECTIVE
To optimize the MR imaging protocol for coronary arterial wall depiction in vitro and characterize the coronary atherosclerotic plaques.
MATERIALS AND METHODS
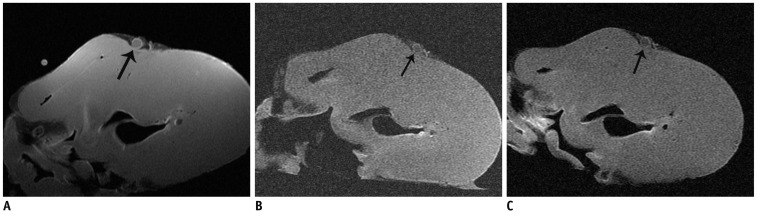
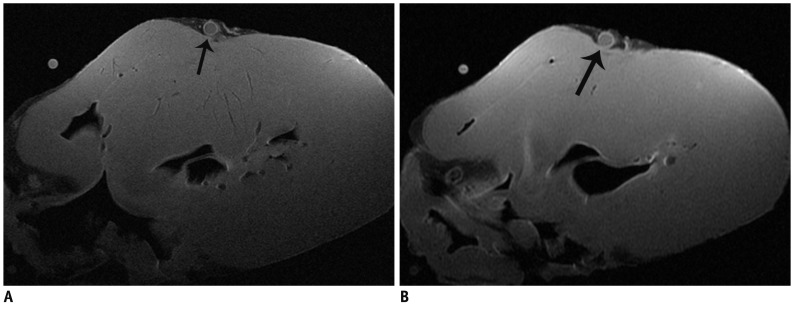
MRI examination was prospectively performed in ten porcine hearts in order to optimize the MR imaging protocol. Various surface coils were used for coronary arterial wall imaging with the same parameters. Then, the image parameters were further optimized for high-resolution coronary wall imaging. The signal-noise ratio (SNR) and contrast-noise ratio (CNR) of images were measured. Finally, 8 human cadaver hearts with coronary atherosclerotic plaques were prospectively performed with MRI examination using optimized protocol in order to characterize the coronary atherosclerotic plaques.
RESULTS
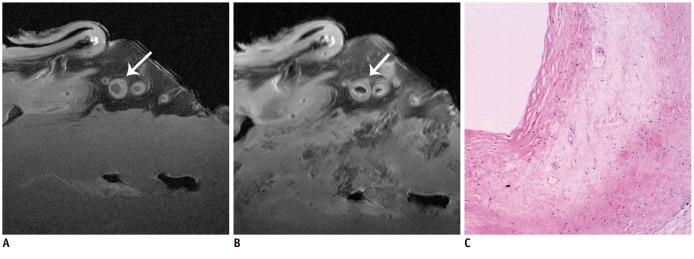
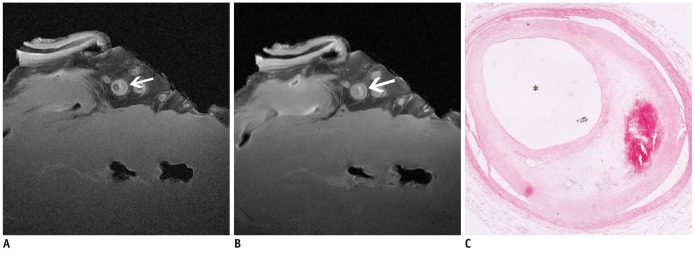
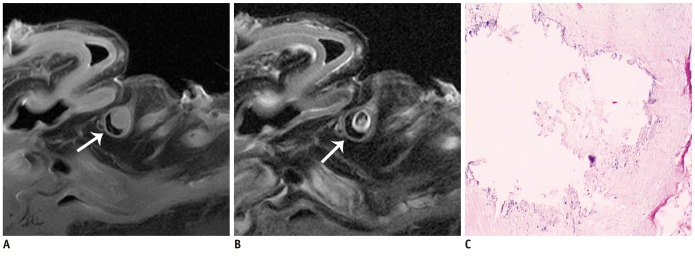
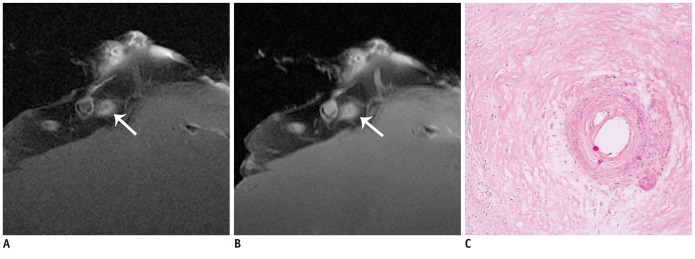
The SNR and CNR of MR image with temporomandibular coil were the highest of various surface coils. High-resolution and high SNR and CNR for ex vivo coronary artery wall depiction can be achieved using temporomandibular coil with 512 x 512 in matrix. Compared with histopathology, the sensitivity and specificity of MRI for identifying advanced plaques were: type IV-V (lipid, necrosis, fibrosis), 94% and 95%; type VI (hemorrhage), 100% and 98%; type VII (calcification), 91% and 100%; and type VIII (fibrosis without lipid core), 100% and 98%, respectively.
CONCLUSION
Temporomandibular coil appears to be dramatically superior to eight-channel head coil and knee coil for ex vivo coronary artery wall imaging, providing higher spatial resolution and improved the SNR. Ex vivo high-resolution MRI has capability to distinguish human coronary atherosclerotic plaque compositions and accurately classify advanced plaques.
MeSH Terms
Figure
Reference
-
1. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995; 92:657–671. PMID: 7634481.
Article2. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47(8 Suppl):C13–C18. PMID: 16631505.
Article3. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992; 326:242–250. PMID: 1727977.4. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992; 326:310–318. PMID: 1728735.5. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20:1262–1275. PMID: 10807742.6. Nikolaou K, Becker CR, Flohr T, Huber A, Scheidler J, Fayad ZA, et al. Optimization of ex vivo CT- and MR- imaging of atherosclerotic vessel wall changes. Int J Cardiovasc Imaging. 2004; 20:327–334. PMID: 15529917.
Article7. Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W Jr, Richardson M, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992; 85:391–405. PMID: 1728483.
Article8. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001; 104:2051–2056. PMID: 11673345.
Article9. Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging. 2002; 15:62–67. PMID: 11793458.
Article10. Fayad ZA, Nahar T, Fallon JT, Goldman M, Aguinaldo JG, Badimon JJ, et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation. 2000; 101:2503–2509. PMID: 10831525.11. Gweon HM, Kim SJ, Lee SM, Hong YJ, Kim TH. 3D whole-heart coronary MR angiography at 1.5T in healthy volunteers: comparison between unenhanced SSFP and Gd-enhanced FLASH sequences. Korean J Radiol. 2011; 12:679–685. PMID: 22043149.
Article12. Kim WY, Astrup AS, Stuber M, Tarnow L, Falk E, Botnar RM, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007; 115:228–235. PMID: 17190865.
Article13. Macedo R, Chen S, Lai S, Shea S, Malayeri AA, Szklo M, et al. MRI detects increased coronary wall thickness in asymptomatic individuals: the multi-ethnic study of atherosclerosis (MESA). J Magn Reson Imaging. 2008; 28:1108–1115. PMID: 18837001.
Article14. Gerretsen S, Kessels AG, Nelemans PJ, Dijkstra J, Reiber JH, van der Geest RJ, et al. Detection of coronary plaques using MR coronary vessel wall imaging: validation of findings with intravascular ultrasound. Eur Radiol. 2013; 23:115–124. PMID: 22782568.
Article15. Serfaty JM, Chaabane L, Tabib A, Chevallier JM, Briguet A, Douek PC. Atherosclerotic plaques: classification and characterization with T2-weighted high-spatial-resolution MR imaging-- an in vitro study. Radiology. 2001; 219:403–410. PMID: 11323464.16. Yuan C, Hatsukami TS, Obrien KD. High-Resolution magnetic resonance imaging of normal and atherosclerotic human coronary arteries ex vivo: discrimination of plaque tissue components. J Investig Med. 2001; 49:491–499.17. Li T, Li X, Zhao X, Zhou W, Cai Z, Yang L, et al. Classification of human coronary atherosclerotic plaques using ex vivo high-resolution multicontrast-weighted MRI compared with histopathology. AJR Am J Roentgenol. 2012; 198:1069–1075. PMID: 22528895.
Article18. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002; 106:1368–1373. PMID: 12221054.
Article19. Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994; 89:2462–2478. PMID: 8181179.
Article20. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995; 92:1355–1374. PMID: 7648691.21. Nikolaou K, Becker CR, Muders M, Babaryka G, Scheidler J, Flohr T, et al. Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis. 2004; 174:243–252. PMID: 15136054.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-Resolution Magnetic Resonance Imaging of Intracranial Vertebral Artery Dissecting Aneurysm for Planning of Endovascular Treatment
- Vessel Wall Imaging of the Intracranial and Cervical Carotid Arteries
- High-Resolution MRI of Intracranial Atherosclerotic Disease
- One-stop Diagnosis of Ischemic Heart Disease Using Cardiac MRI
- Noninvasive Imaging of Atherosclerotic Plaques Using MRI and CT