J Korean Med Sci.
2010 May;25(5):738-745. 10.3346/jkms.2010.25.5.738.
Treatment Outcomes of Clevudine versus Lamivudine at Week 48 in Naive Patients with HBeAg Positive Chronic Hepatitis B
- Affiliations
-
- 1Department of Internal Medicine, Chonbuk National University Medical School and Hospital, Jeonju, Korea. daeghon@chonbuk.ac.kr
- 2The Research Institute for Medical Science, Chonbuk National University Medical School and Hospital, Jeonju, Korea.
- 3Department of Internal Medicine, Wonkwang University College of Medicine and Hospital, Iksan, Korea.
- KMID: 1713959
- DOI: http://doi.org/10.3346/jkms.2010.25.5.738
Abstract
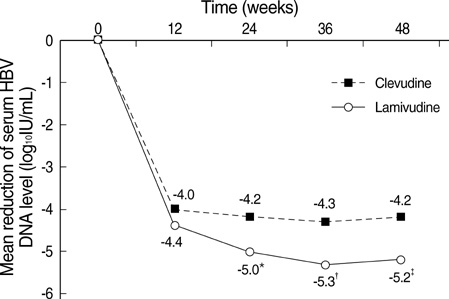
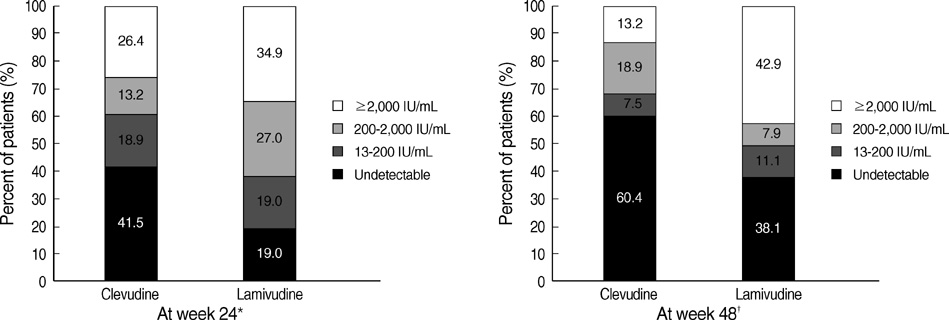
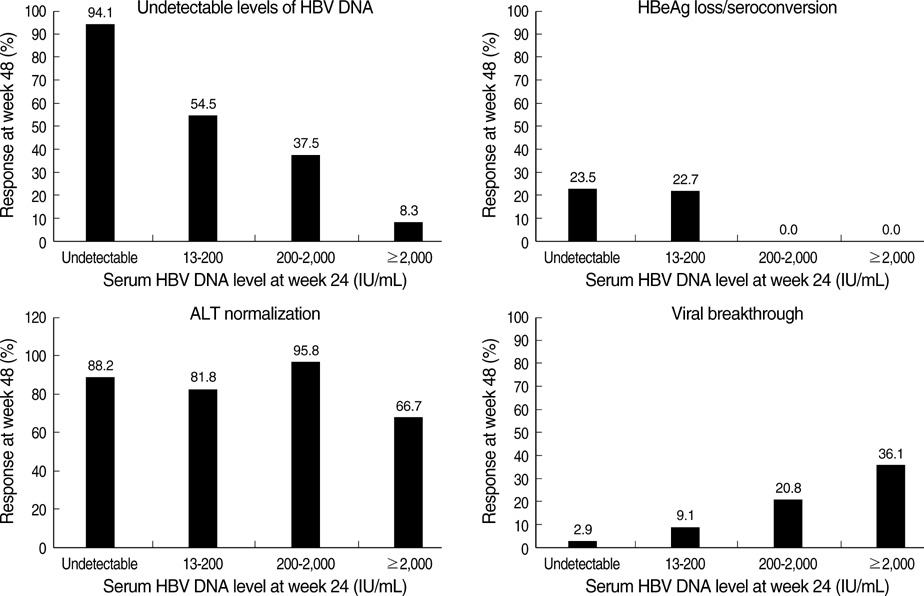
- The authors assessed the efficacy and antiviral resistance of 48-week clevudine therapy versus lamivudine in treatment of naive patients with HBeAg positive chronic hepatitis B. In this retrospective study, a total of 116 HBeAg positive patients, who received 30 mg of clevudine once daily (n=53) or 100 mg of lamivudine once daily (n=63) for 48 weeks, were included. At week 48, clevudine therapy produced a significantly greater mean reductions in serum HBV DNA levels from baseline than lamivudine therapy (-5.2 vs. -4.2 log(10)IU/mL; P=0.005). Furthermore, a significantly higher proportion of patients on clevudine achieved negative serum HBV DNA by PCR (<13 IU/mL) at week 48 (60.4% vs. 38.1%; P=0.025). The incidence of virologic breakthrough in the clevudine group was significantly lower than in the lamivudine group (9.4% vs. 25.4%; P=0.031). However, rates of alanine aminotransferase normalization and HBeAg loss or seroconversion were similar in the two groups (83.0% vs. 81.0%, 11.3% vs. 11.1%; P=0.813, 1.000, respectively). In conclusion, clevudine is more potent for viral suppression and lower for antiviral resistance at week 48 than lamivudine in treatment of naive patients with HBeAg positive chronic hepatitis B.
Keyword
MeSH Terms
-
Adult
Antiviral Agents/administration & dosage
Arabinofuranosyluracil/administration & dosage/*analogs & derivatives
Drug Resistance, Viral
Female
Hepatitis B e Antigens/*blood
Hepatitis B, Chronic/diagnosis/*drug therapy/*immunology
Humans
Lamivudine/*administration & dosage
Male
Treatment Outcome
Antiviral Agents
Hepatitis B e Antigens
Lamivudine
Arabinofuranosyluracil
Figure
Reference
-
1. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007. 45:507–539.
Article2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009. 50:227–242.3. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008. 6:1315–1341.
Article4. Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther. 2004. 9:1013–1026.5. Balakrishna Pai S, Liu SH, Zhu YL, Chu CK, Cheng YC. Inhibition of hepatitis B virus by a novel L-nucleoside, 2'-fluoro-5-methyl-beta-L-arabinofuranosyl uracil. Antimicrob Agents Chemother. 1996. 40:380–386.
Article6. Chin R, Shaw T, Torresi J, Sozzi V, Trautwein C, Bock T, Manns M, Isom H, Furman P, Locarnini P. In vitro susceptibilities of wild-type or drug-resistant hepatitis B virus to (-)-beta-D-2,6-diaminopurine dioxolane and 2'-fluoro-5-methyl-beta-L-arabinofuranosyluracil. Antimicrob Agents Chemother. 2001. 45:2495–2501.7. Marcellin P, Mommeja-Marin H, Sacks SL, Lau GK, Sereni D, Bronowicki JP, Conway B, Trepo C, Blum MR, Yoo BC, Mondou E, Sorbel J, Snow A, Rousseau F, Lee HS. A phase II dose-escalating trial of clevudine in patients with chronic hepatitis B. Hepatology. 2004. 40:140–148.
Article8. Lee HS, Chung YH, Lee K, Byun KS, Paik SW, Han JY, Yoo K, Yoo HW, Lee JH, Yoo BC. A 12-week clevudine therapy showed potent and durable antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2006. 43:982–988.
Article9. Lim SG, Leung N, Hann HW, Lau GK, Trepo C, Mommeja-Marin H, Moxham C, Sorbel J, Snow A, Blum MR, Rousseau F, Marcellin P. Clinical trial: a phase II, randomized study evaluating the safety, pharmacokinetics and anti-viral activity of clevudine for 12 weeks in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2008. 27:1282–1292.10. Yoo BC, Kim JH, Kim TH, Koh KC, Um SH, Kim YS, Lee KS, Han BH, Chon CY, Han JY, Ryu SH, Kim HC, Byun KS, Hwang SG, Kim BI, Cho M, Yoo K, Lee HJ, Hwang JS, Kim YS, Lee YS, Choi SK, Lee YJ, Yang JM, Park JW, Lee MS, Kim DG, Chung YH, Cho SH, Choi JY, Kweon YO, Lee HY, Jeong SH, Yoo HW, Lee HS. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology. 2007. 46:1041–1048.
Article11. Yoo BC, Kim JH, Chung YH, Lee KS, Paik SW, Ryu SH, Han BH, Han JY, Byun KS, Cho M, Lee HJ, Kim TH, Cho SH, Park JW, Um SH, Hwang SG, Kim YS, Lee YJ, Chon CY, Kim BI, Lee YS, Yang JM, Kim HC, Hwang JS, Choi SK, Kweon YO, Jeong SH, Lee MS, Choi JY, Kim DG, Kim YS, Lee HY, Yoo K, Yoo HW, Lee HS. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2007. 45:1172–1178.
Article12. Saldanha J, Gerlich W, Lelie N, Dawson P, Heermann K, Heath A. WHO Collaborative Study Group. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 2001. 80:63–71.
Article13. Gish RG, Locarnini SA. Chronic hepatitis B: current testing strategies. Clin Gastroenterol Hepatol. 2006. 4:666–676.
Article14. Hong SP, Kim NK, Hwang SG, Chung HJ, Kim S, Han JH, Kim HT, Rim KS, Kang MS, Yoo W, Kim SO. Detection of hepatitis B virus YMDD variants using mass spectrometric analysis of oligonucleotide fragments. J Hepatol. 2004. 40:837–844.15. Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C. Hepatitis B Virus Drug Resistance Working Group. Antiviral drug-resistant HBV: Standardization of nomenclature and assays and recommendations for management. Hepatology. 2007. 46:254–265.
Article16. Lee HJ, Eun JR, Lee CH, Hwang JS, Suh JI, Kim BS, Jang BK. Long-term clevudine therapy in nucleos(t)ide-naïve and lamivudine-experienced patients with hepatitis B virus-related chronic liver diseases. Korean J Hepatol. 2009. 15:179–192.
Article17. Yang H, Qi X, Sabogal A, Miller M, Xiong S, Delaney WE 4th. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir Ther. 2005. 10:625–633.18. Lampertico P, Viganó M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007. 133:1445–1451.
Article19. Manolakopoulos S, Bethanis S, Koutsounas S, Goulis J, Vlachogiannakos J, Christias E, Saveriadis A, Pavlidis C, Triantos C, Christidou A, Papatheodoridis G, Karamanolis D, Tzourmakliotis D. Long-term therapy with adefovir dipivoxil in hepatitis B e antigen-negative patients developing resistance to lamivudine. Aliment Pharmacol Ther. 2008. 27:266–273.
Article20. van Bömmel F, Zollner B, Sarrazin C, Spengler U, Hüppe D, Müller B, Feucht HH, Wiedenmann B, Berg T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006. 44:318–325.
Article21. Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R, Cross A, Denisky G, Kreter B, Hindes R. AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006. 130:2039–2049.
Article22. Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, Han S, Poynard T, Myers M, Chao G, Lloyd D, Brown NA. Telbivudine Phase II Investigator Group. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005. 129:528–536.
Article23. Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J, Han SH, Hwang SG, Cakaloglu Y, Tong MJ, Papatheodoridis G, Chen Y, Brown NA, Albanis E, Galil K, Naoumov NV. GLOBE Study Group. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009. 136:486–495.
Article24. Nguyen MH, Keeffe EB. Chronic hepatitis B: early viral suppression and long-term outcomes of therapy with oral nucleos(t)ides. J Viral Hepat. 2009. 16:149–155.
Article25. Seok JI, Lee DK, Lee CH, Park MS, Kim SY, Kim HS, Jo HY, Lee CH, Kim DS. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009. 49:2080–2086.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Forty-eight weeks treatment with clevudine 30 mg qd versus lamivudine 100 mg qd for chronic hepatitis B infection: a double-blind randomized study
- Treatment Efficacy of Clevudine, Entecavir and Lamivudine in Treatment-naive Patients with HBeAg-Positive Chronic Hepatitis B
- Efficacy of 48-week clevudine therapy for chronic hepatitis B
- The management and treatment of chronic hepatitis B in Korean children
- Development of Clevudine Resistance after Switching from Lamivudine in a Patient with Chronic Hepatitis B